Korean J Ophthalmol.
2011 Jun;25(3):196-201. 10.3341/kjo.2011.25.3.196.
Mechanisms of Apoptosis on Human Lens Epithelium after Ultraviolet Light Exposure
- Affiliations
-
- 1Department of Ophthalmology, Chosun University School of Medicine, Gwangju, Korea. clearcornea@paran.com
- KMID: 1010028
- DOI: http://doi.org/10.3341/kjo.2011.25.3.196
Abstract
- PURPOSE
The purpose of this study is to understand the mechanism of apoptosis occurring on a cultured human lens epithelial cell line after exposure to ultraviolet (UV) light. We intended to confirm the presence of cellular toxicity and apoptosis and to reveal the roles of p53, caspase 3 and NOXA in these processes.
METHODS
Cells were irradiated with an ultraviolet lamp. Cellular toxicity was measured by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Hoechst staining and fluorescent anti-caspase 3 antibodies were used for apoptosis investigation. The quantities of p53, caspase 3, and NOXA were measured by Western blotting for to investigate the apoptosis pathway.
RESULTS
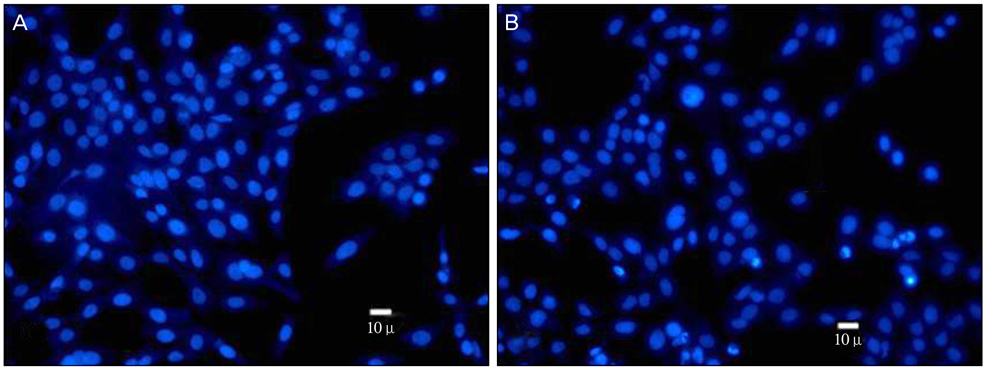
Cellular toxicity on the human lens epithelium markedly increased with time after UV exposure. On Hoechst staining, we found that apoptosis also remarkably increased after exposure to ultraviolet light, compared with a control group. In the immunochemical study using anti-caspase 3 antibodies, active caspase 3 significantly increased after exposure to ultraviolet light. On Western blotting, p53 decreased, while caspase 3 and NOXA increased.
CONCLUSIONS
Exposure of cultured human lens epithelial cell lines to ultraviolet light induces apoptosis, which promotes the expression of NOXA and caspase 3 increases without increasing p53. This may suggest that UV induced apoptosis is caused by a p53-independent pathway in human lens epithelial cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Hightower KR. The role of the lens epithelium in development of UV cataract. Curr Eye Res. 1995. 14:71–78.2. Sasaki K. Matthes R, editor. International Commission on Non-Ionizing Radiation Protection. International Commission on Illumination. The lens-human data from chronic exposure: UV related cataract. Measurements of optical radiation hazards. 1998. Oberschleissheim: International Commission on Non-Ionizing Radiation Protection;179–192.3. Taylor HR, West SK, Rosenthal FS, et al. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988. 319:1429–1433.4. Kleiman NJ, Wang RR, Spector A. Ultraviolet light induced DNA damage and repair in bovine lens epithelial cells. Curr Eye Res. 1990. 9:1185–1193.5. Kojima M, Yamada Y, Sasaki K. Photo-damage and the repair process of lens epithelia cells induced by a single exposure ultraviolet light. Invest Ophthalmol Vis Sci. 1999. 40:B664.6. Ayala M, Strid H, Jacobsson U, et al. p53 expression and apoptosis in the lens after ultraviolet radiation exposure. Invest Ophthalmol Vis Sci. 2007. 48:4187–4191.7. Andersson M, Honarvar A, Sjostrand J, et al. Decreased caspase-3 activity in human lens epithelium from posterior subcapsular cataracts. Exp Eye Res. 2003. 76:175–182.8. Shibue T, Takeda K, Oda E, et al. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003. 17:2233–2238.9. Li WC, Spector A. Lens epithelial cell apoptosis is an early event in the development of UVB-induced cataract. Free Radic Biol Med. 1996. 20:301–311.10. Godar DE. Preprogrammed and programmed cell death mechanisms of apoptosis: UV-induced immediate and delayed apoptosis. Photochem Photobiol. 1996. 63:825–830.11. Hightower KR, Reddan JR, McCready JP, Dziedzic DC. Lens epithelium: a primary target of UVB irradiation. Exp Eye Res. 1994. 59:557–564.12. Tamada Y, Fukiage C, Nakamura Y, et al. Evidence for apoptosis in the selenite rat model of cataract. Biochem Biophys Res Commun. 2000. 275:300–306.13. Worgul BV, Merriam GR Jr, Medvedovsky C. Cortical cataract development: an expression of primary damage to the lens epithelium. Lens Eye Toxic Res. 1989. 6:559–571.14. Hightower K, McCready J. Physiological effects of UVB irradiation on cultured rabbit lens. Invest Ophthalmol Vis Sci. 1992. 33:1783–1787.15. Zigman S, Vaughan T. Near-ultraviolet light effects on the lenses and retinas of mice. Invest Ophthalmol. 1974. 13:462–465.16. Hightower K, McCready J. Comparative effect of UVA and UVB on cultured rabbit lens. Photochem Photobiol. 1993. 58:827–830.17. Harding JJ, Crabbe MJ. Davson H, editor. The lens: development, proteins, metabolism and cataract. The eye. 1984. Vol. 1B. London: Academic Press;207–492.18. Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001. 29(Pt 6):684–688.19. Geatrell JC, Gan PM, Mansergh FC, et al. Apoptosis gene profiling reveals spatio-temporal regulated expression of the p53/Mdm2 pathway during lens development. Exp Eye Res. 2009. 88:1137–1151.20. Ayala M, Strid H, Jacobsson U, Soderberg PG. p53 expression and apoptosis in the lens after ultraviolet radiation exposure. Invest Ophthalmol Vis Sci. 2007. 48:4187–4191.21. Pokroy R, Tendler Y, Pollack A, et al. p53 expression in the normal murine eye. Invest Ophthalmol Vis Sci. 2002. 43:1736–1741.22. Hall PA, McKee PH, Menage HD, et al. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993. 8:203–207.23. Villunger A, Michalak EM, Coultas L, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003. 302:1036–1038.24. Bennett M, Macdonald K, Chan SW, et al. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998. 282:290–293.25. Grabner G, Brenner W. Unscheduled DNA repair in human lens epithelium following 'in vivo' and 'in vitro' ultraviolet irradiation. Ophthalmic Res. 1982. 14:160–166.26. Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003. 4:321–328.27. Oda E, Ohki R, Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000. 288:1053–1058.28. Qin JZ, Stennett L, Bacon P, et al. p53-independent NOXA induction overcomes apoptotic resistance of malignant melanomas. Mol Cancer Ther. 2004. 3:895–902.29. Jullig M, Zhang WV, Ferreira A, Stott NS. MG132 induced apoptosis is associated with p53-independent induction of pro-apoptotic Noxa and transcriptional activity of beta-catenin. Apoptosis. 2006. 11:627–641.30. Armstrong JL, Veal GJ, Redfern CP, Lovat PE. Role of Noxa in p53-independent fenretinide-induced apoptosis of neuroectodermal tumours. Apoptosis. 2007. 12:613–622.31. Banerjee G, Gupta N, Kapoor A, Raman G. UV induced bystander signaling leading to apoptosis. Cancer Lett. 2005. 223:275–284.32. Singleton KR, Will DS, Schotanus MP, et al. Elevated extracellular K+ inhibits apoptosis of corneal epithelial cells exposed to UV-B radiation. Exp Eye Res. 2009. 89:140–151.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of L-ascorbate on Cell Death Induced by Ultraviolet A in Ciliary Body Epithelium
- Comparative Spectrophotometer Analysis of Ultraviolet-light Filtering, Blue-light Filtering, and Violet-light Filtering Intraocular Lenses
- An Experimental Study for Sunscreening Effect of Several Sunscreen Agents
- Apoptosis Induced by Contact Lens Wearing in Rabbit Cornea
- Ultraviolet B-Induced Apoptosis of Normal Human Melanocytes and G361 Cells