Korean J Ophthalmol.
2005 Sep;19(3):213-218. 10.3341/kjo.2005.19.3.213.
Morphological Characteristics and Intercellular Connections of Corneal Keratocytes
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Hanyang University, Seoul, Korea. fovea@hanyang.ac.kr
- KMID: 754427
- DOI: http://doi.org/10.3341/kjo.2005.19.3.213
Abstract
- PURPOSE
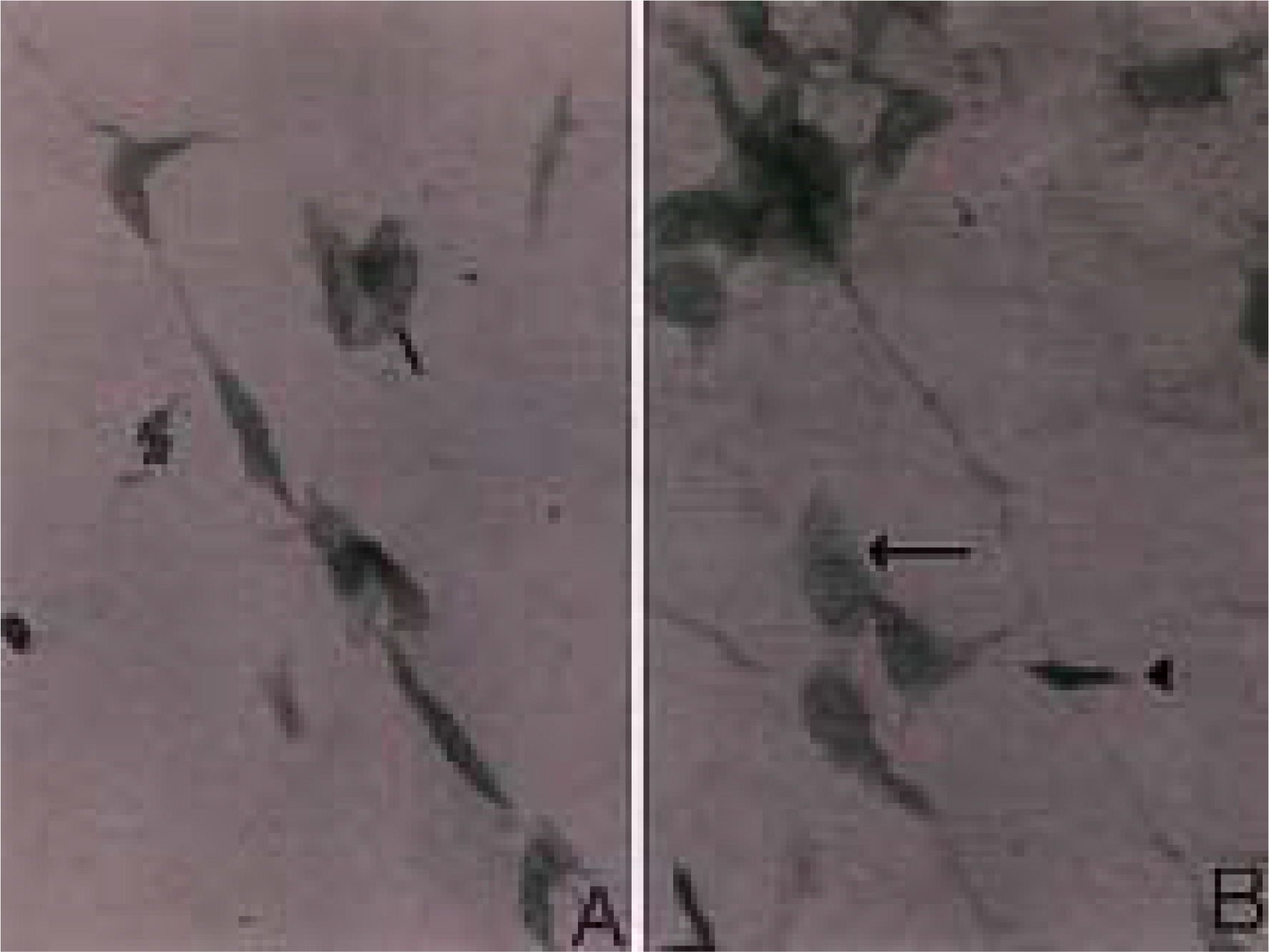
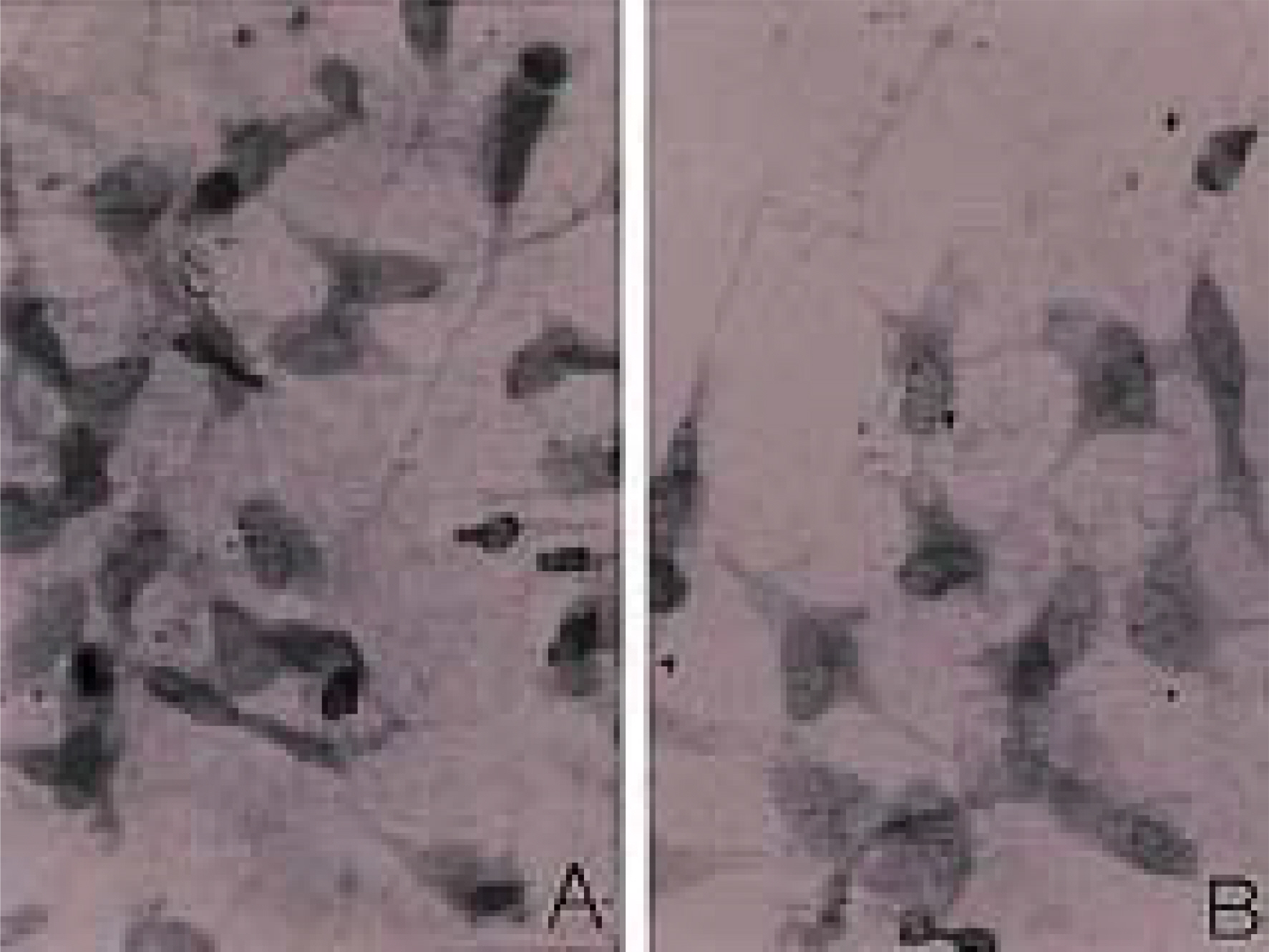
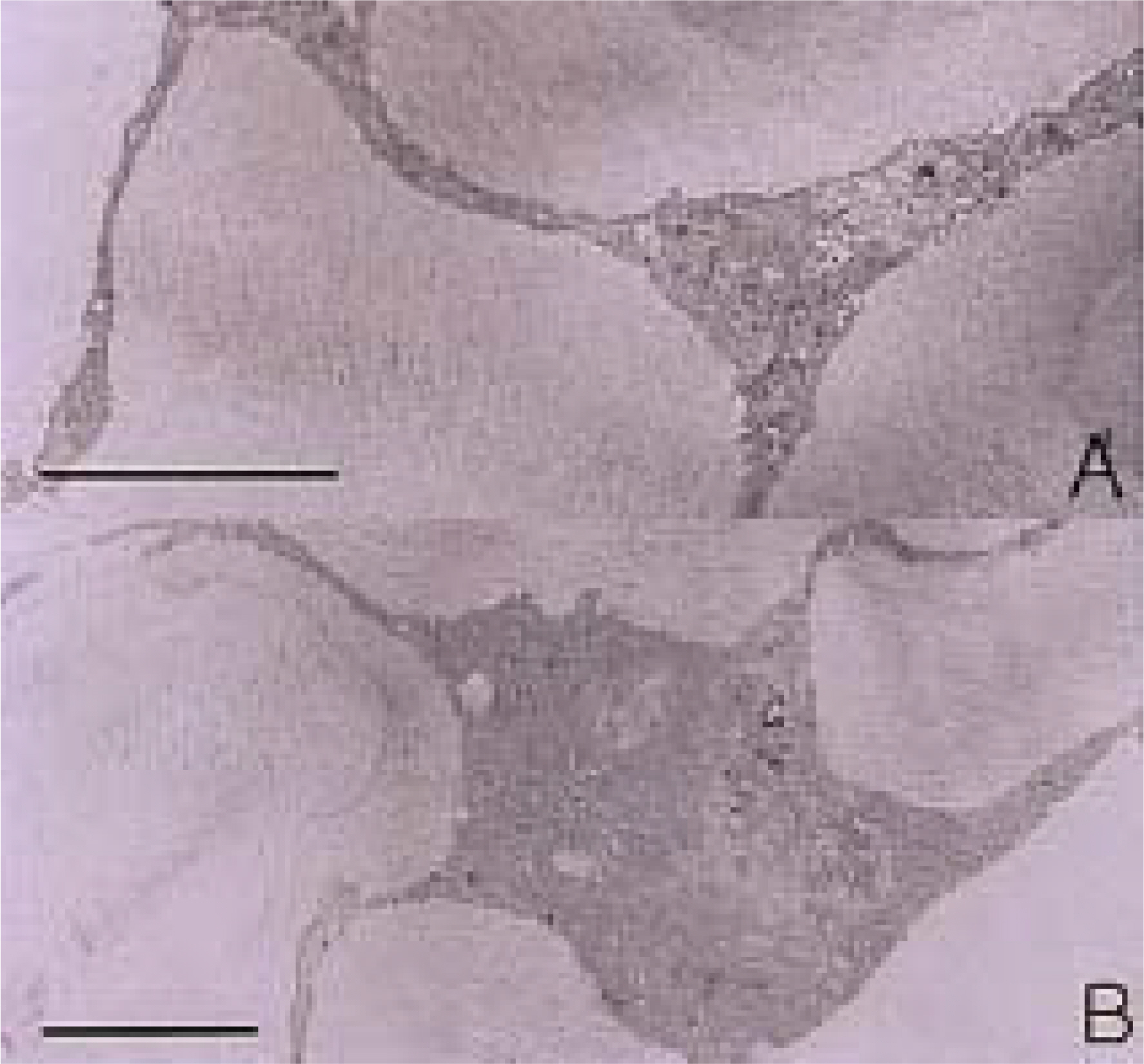
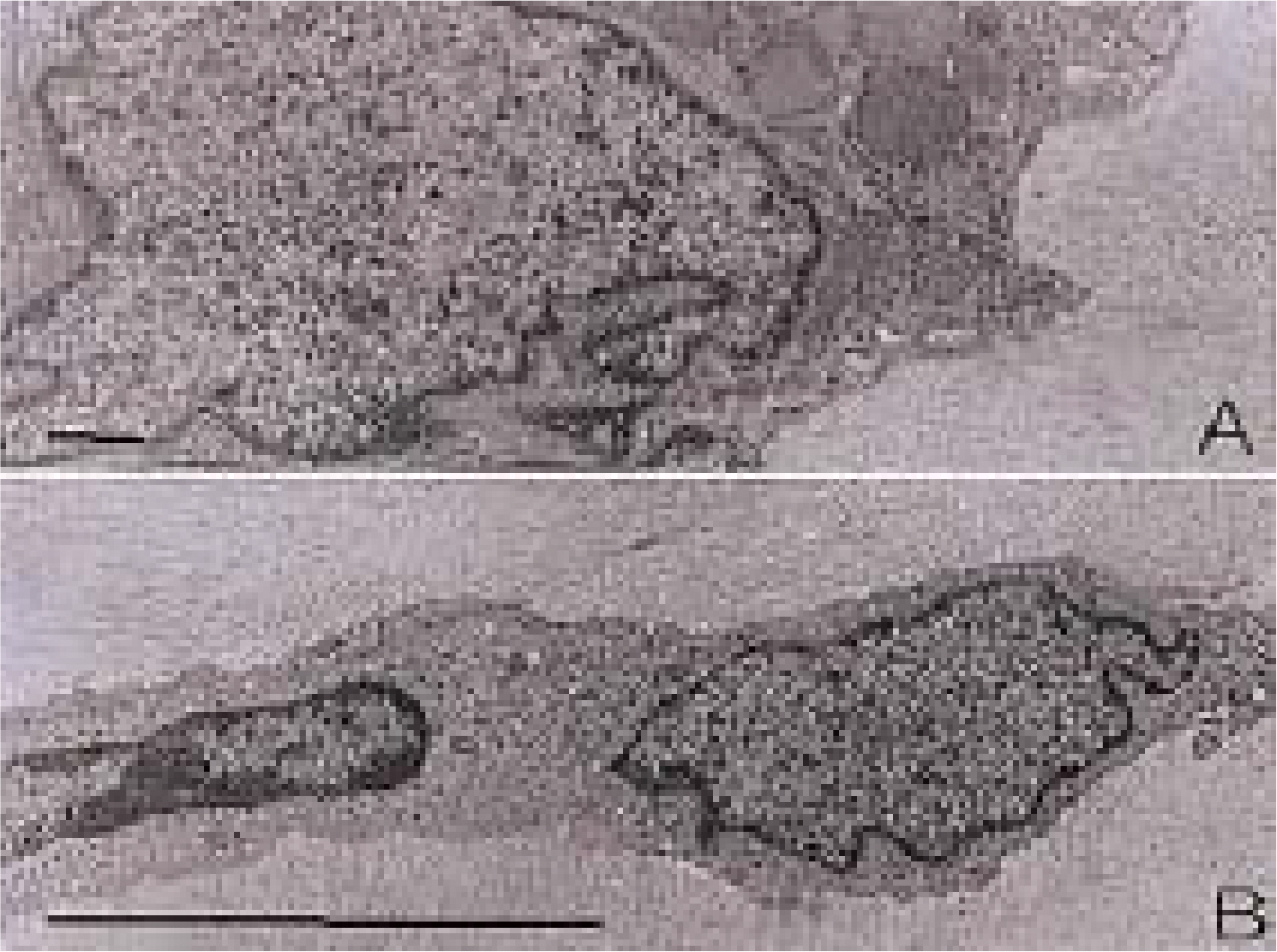
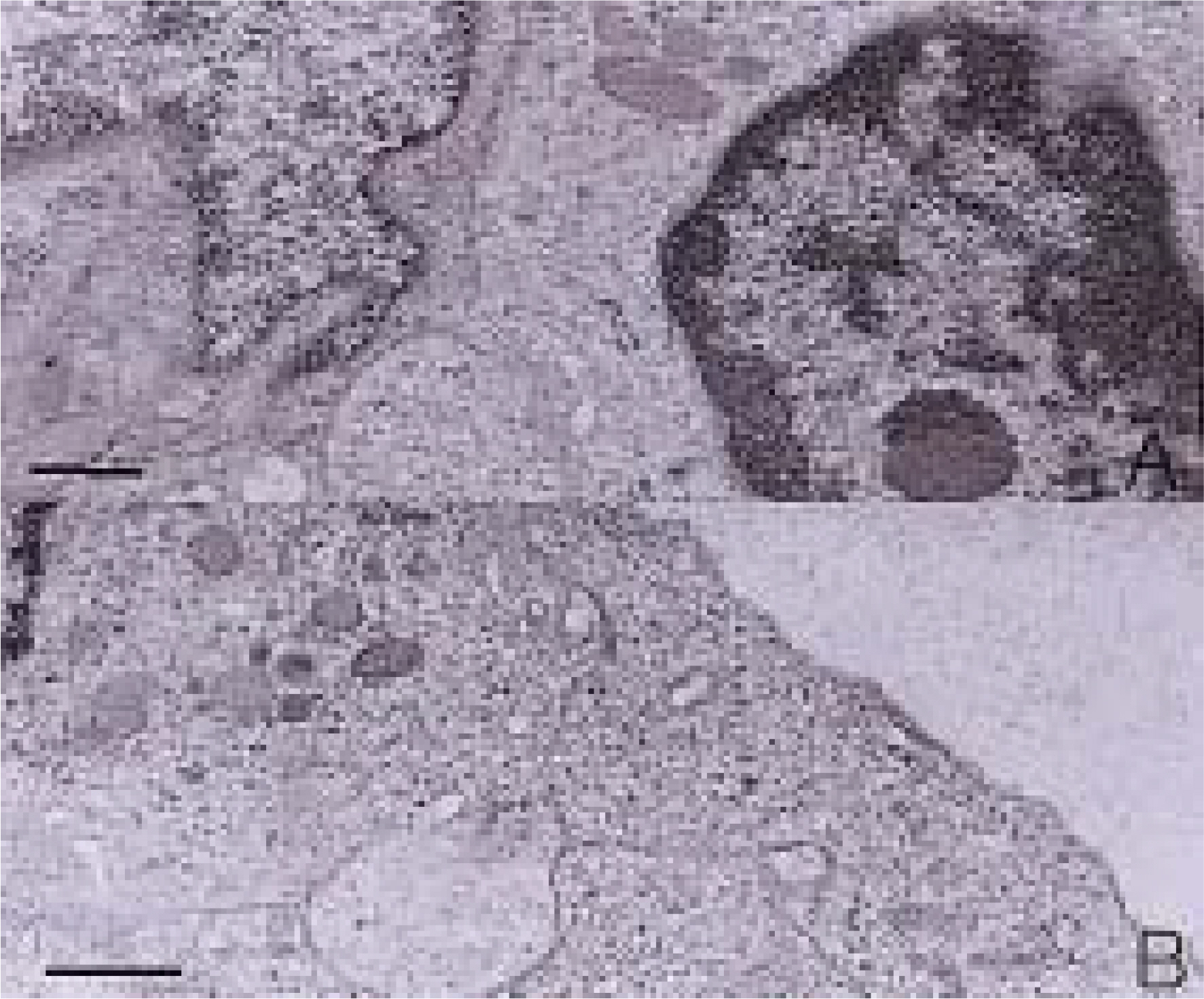
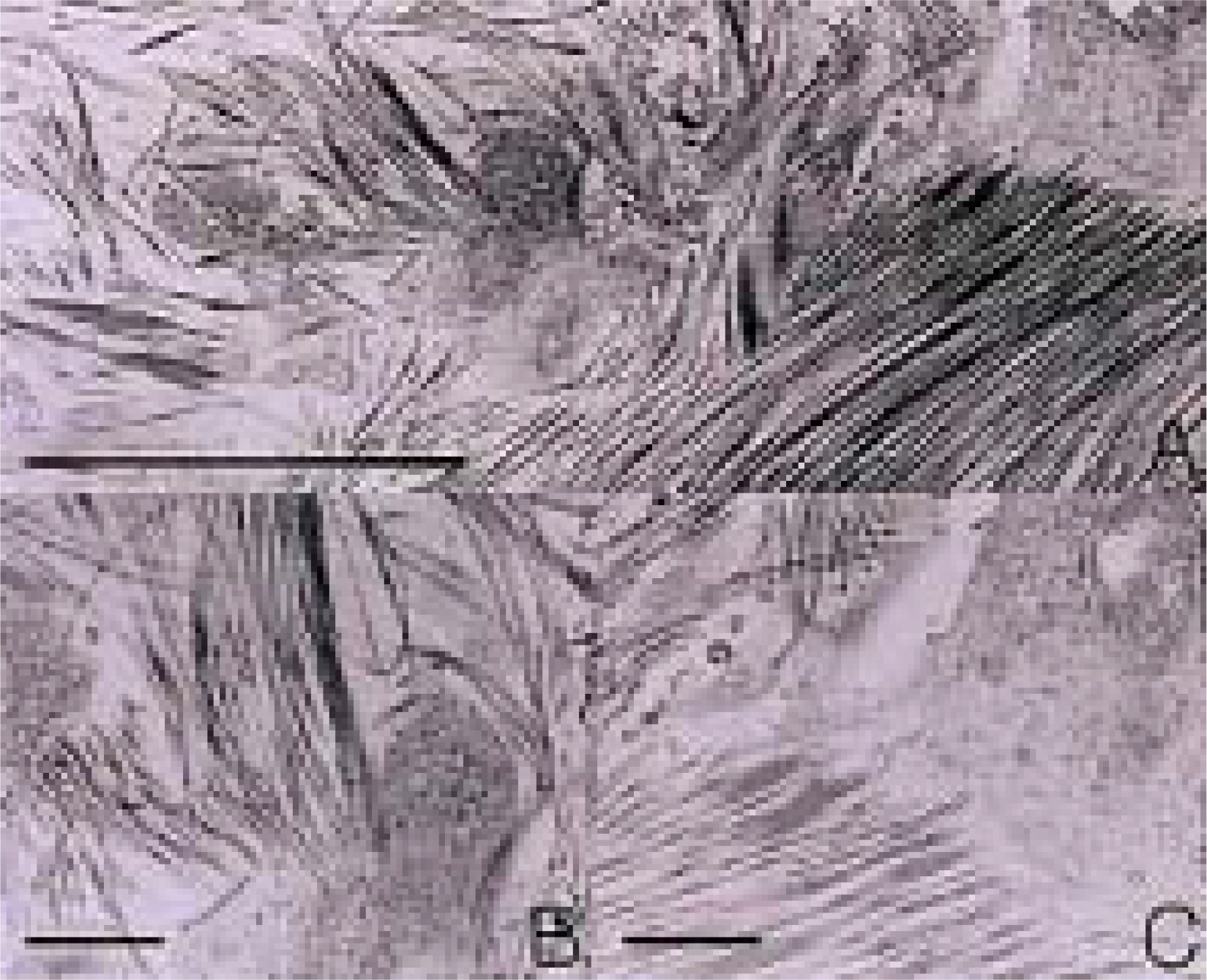
To investigate the morphological characteristics of keratocytes and the interconnection of keratocytes with adjacent keratocytes using the flat preparation method and scanning electron microscopy with a frontal section of the human corneal stroma. METHODS: The thin, corneal collagen lamellae were carefully dissected from the cornea (n=7), which had been stained by the flat preparation method. The remaining tissue was fixed in 3% glutaraldehyde and observed by transmission electron microscopy following the frontal section. RESULTS: The flat preparation revealed the corneal fibroblasts between the lamellae of the collagen fibers and showed that the ramifying cellular processes of the keratocytes were in contact with the cytoplasmic processes or cell bodies of neighboring fibroblasts. Two types of discrete subpopulations of keratocytes were identified: a smaller, cellular type of keratocyte with spindle-shaped nucleus with heterochromatin, and a larger, cellular type with a large indented nucleus with relatively scanty cytoplasm. Collagen fibers ran parallel to each other toward the fenestration of the cytoplasmic wall of the keratocyte. CONCLUSIONS: These flat preparation method results showed that the keratocytes within the corneal stroma are interconnected with the adjacent keratocytes, which indicates the presence of a functional communicating network through the keratocyte circuits within the stroma. A smaller, cellular type of keratocyte with spindle-shaped nucleus was morphologically differentiated from a larger, cellular type with a large, indented nucleus by flat preparation and transmission electron microscopy.
MeSH Terms
Figure
Reference
-
1. Hogan MJ, Alvarado JA. Histology of the Human Eye. Weddell J, editor. The cornea: An Atlas and Textbook. 1st ed.Philadelphia: Saunders;1971. chap. 3.2. Moller-Pedersen T, Ledet T, Ehlers N. The keratocyte density of human donor corneas. Curr Eye Res. 1994; 13:163–9.3. Maurice DM. The structure and transparency of the cornea. J Physiol. 1957; 136:263–86.
Article4. Goldman JN, Dohlman GB, Benedek CH, Kravitt B. Structural alterations affecting transparency in swollen human corneas. Invest Ophthalmol Vis Sci. 1968; 7:501–19.5. Farrell RA, McCally RL, Tatham PER. Wavelength dependencies of light scattering in normal and cold swollen rabbit corneas and their structural implications. J Physiol. 1973; 233:589–612.
Article6. Ameen DB, Bishop MF, McMullen T. A lattice model for computing the transmissivity of the cornea and sclera. J Biophys. 1998; 75:2520–31.
Article7. Muller LJ, Pels E, Schurmans LR, Vrensen GF. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp Eye Res. 2004; 78:493–501.8. Muller LJ, Pels L, Vrensten GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995; 36:2557–67.9. Watsky MA. Keratocyte gap junctional communication in normal and wounded rabbit corneas and human corneas. Invest Ophthalmol Vis Sci. 1995; 36:2568–76.10. Nishida T, Yasumoto K, Otori T, Desaki J. The network structure of corneal fibroblasts in the rat as revealed by scanning electron microscopy. Invest Ophthalmol Vis Sci. 1988; 29:1887–90.11. Nishida T, Ueda A, Fukuda M, et al. Interactions of extracellular collagen and corneal fibroblasts: morphologic and biochemical changes of rabbit corneal cells cultured in a collagen matrix. In Vitro Cell Dev Bio. 1988; 24:1009–14.
Article12. Wilson SE, He YG, Weng J, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996; 62:325–7.
Article13. Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998; 39:276–83.14. Patel SV, McLaren JW, Camp JJ, et al. Automated quantification of keratocyte density by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 1999; 40:320–6.15. Moller-Pedersen T, Ehlers N. A three-dimensional study of the human corneal keratocyte density. Curr Eye Res. 1995; 14:459–64.16. Ojeda JL, Ventosa JA, Piedra S. The three-dimensional microanatomy of the rabbit and human cornea. A chemical and mechanical microdissection-SEM approach. J Anat. 2001; 199:567–76.
Article17. Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991; 32:2244–58.18. Waring GO. III Corneal structure and pathophysiology. In Corneal disorders Clinical diagnosis and management. In: Leibowits HM, ed. Philadelphia: Saunders;1984. p. 3–25.19. Ueda A, Nishida T, Otori T, Fujita H. Uptake of India ink particles and latex beads by corneal fibroblasts. Cell Tissue Res. 1987; 250:251–5.
Article20. Marshall J, Grindle CF. Fine structure of the cornea and its development. Trans Ophthalmol Soc U K. 1978; 98:320–8.21. Doughty MJ, Seabert W, Bergmanson JP, Blocker Y. A descriptive and quantitative study of the keratocytes of the corneal stroma of albino rabbits using transmission electron microscopy. Tissue Cell. 2001; 33:408–22.
Article22. Poole CA, Brookes NH, Clover GM. Keratocyte networks visualized in the living cornea using vital dyes. J Cell Sci. 1993; 106:685–91.23. Giraud JP, Pouliquen Y, Offret G, Payrau P. Statistical morphometric studies in normal human and rabbit corneal stroma. Exp Eye Res. 1975; 21:221–9.
Article24. Stockwell RA. Morphometry of cytoplasmic components of mammalian articular chondrocytes and corneal keratocytes: species and zonal variations of mitochondria in relation to nutrition. J Anat. 1991; 175:251–61.25. Watsky MA, Rae JL. Initial characterization of whole-cell currents from freshly dissociated corneal keratocytes. Curr Eye Res. 1992; 11:127–34.
Article26. Poole CA, Brookes NH, Clover GM. Confocal imaging of the keratocyte network in porcine cornea using the fixable vital dye 5-chloromethylfluorescein diacetate. Curr Eye Res. 1996; 15:165–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cultured Corneal Keratocyte: Scanning and Transmission Electron Microscopic Findings
- The Expression of ICAM-1 by Cytokines and the Effect of Dexamethasone on ICAM-1 Expression in Cultured Keratocytes
- Effects of Damaged Human Corneal Epithelial Cells on Differentiation of Human Mesenchymal Stem Cell
- Gene Microarray Related with Apoptosis in Diabetic OLETF Keratocytes
- Lipopolysaccharide-induced Production of Interleukin-8 by Cultured Human Keratocyte