Korean J Radiol.
2001 Dec;2(4):183-191. 10.3348/kjr.2001.2.4.183.
Diffusion-weighted MR Imaging of Intracerebral Hemorrhage
- Affiliations
-
- 1Department of Diagnostic Radiology, Samsung Medical Center, Sungkyun-kwan University School of Medicine. dgna@smc.samsung.co.kr
- 2Department of Diagnostic Radiology, Masan Samsung Hospital, Sungkyun-kwan University School of Medicine.
- KMID: 754086
- DOI: http://doi.org/10.3348/kjr.2001.2.4.183
Abstract
OBJECTIVE
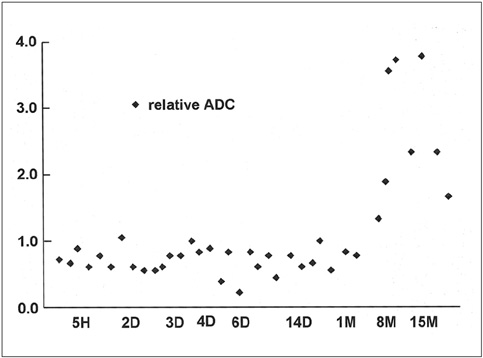
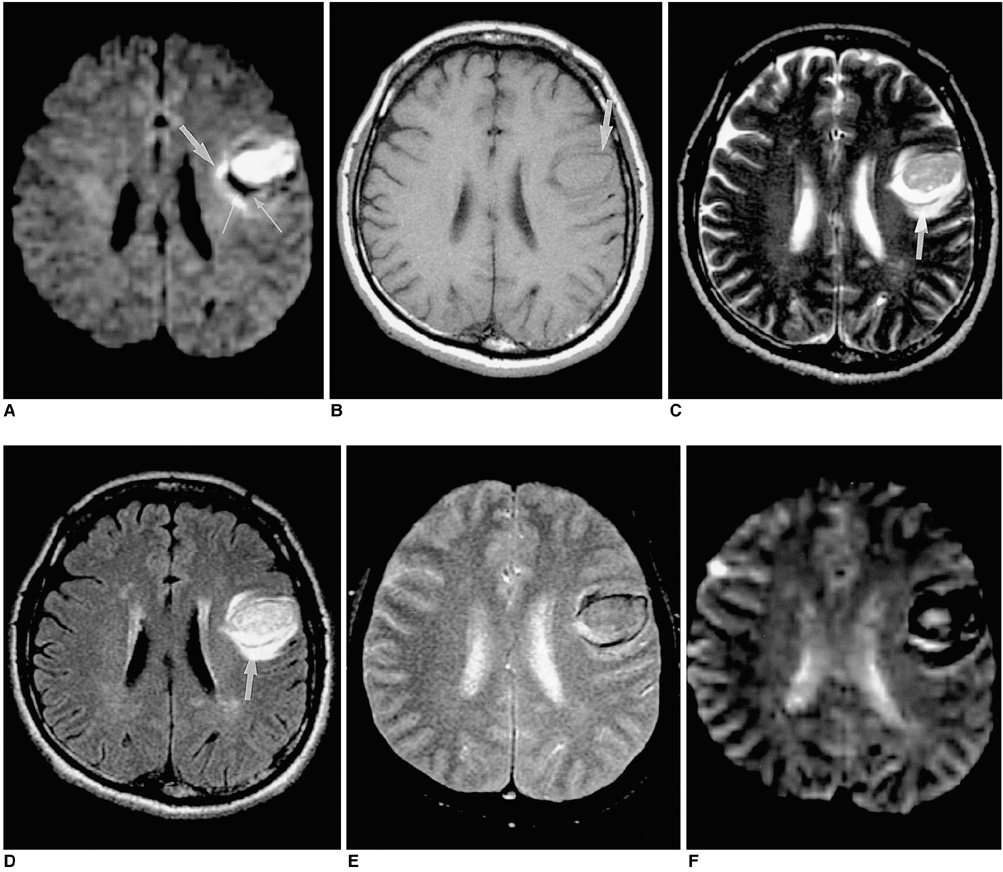
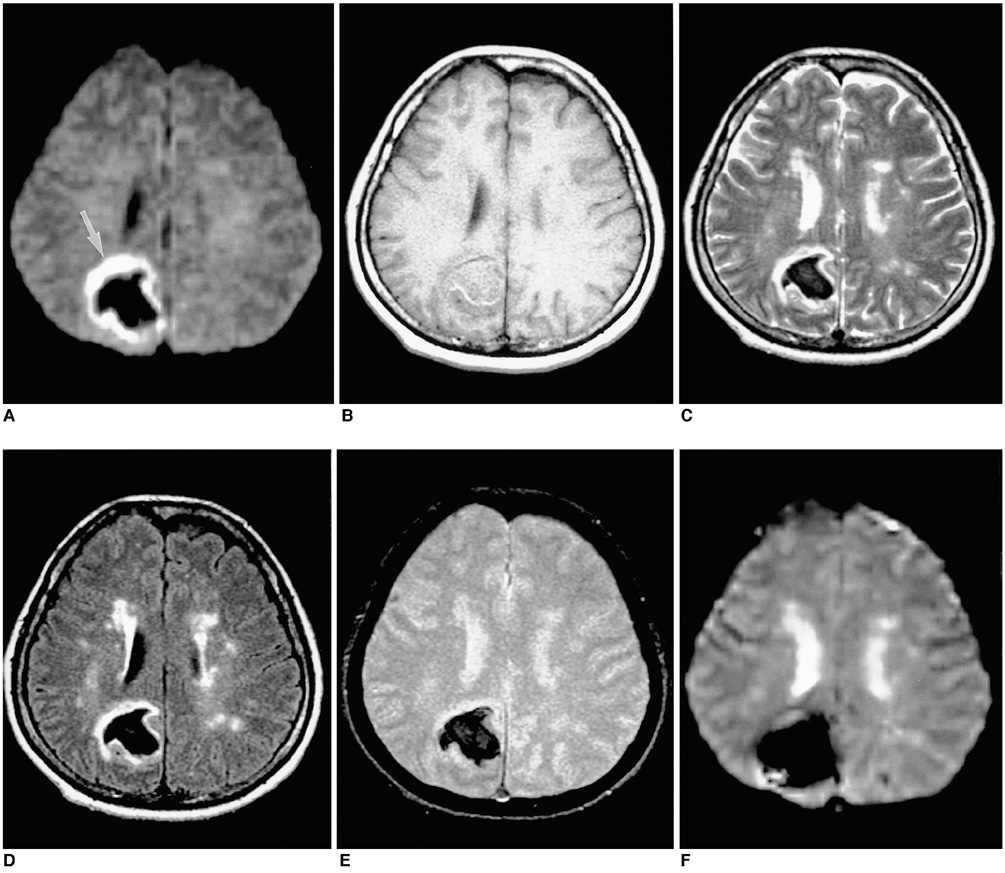
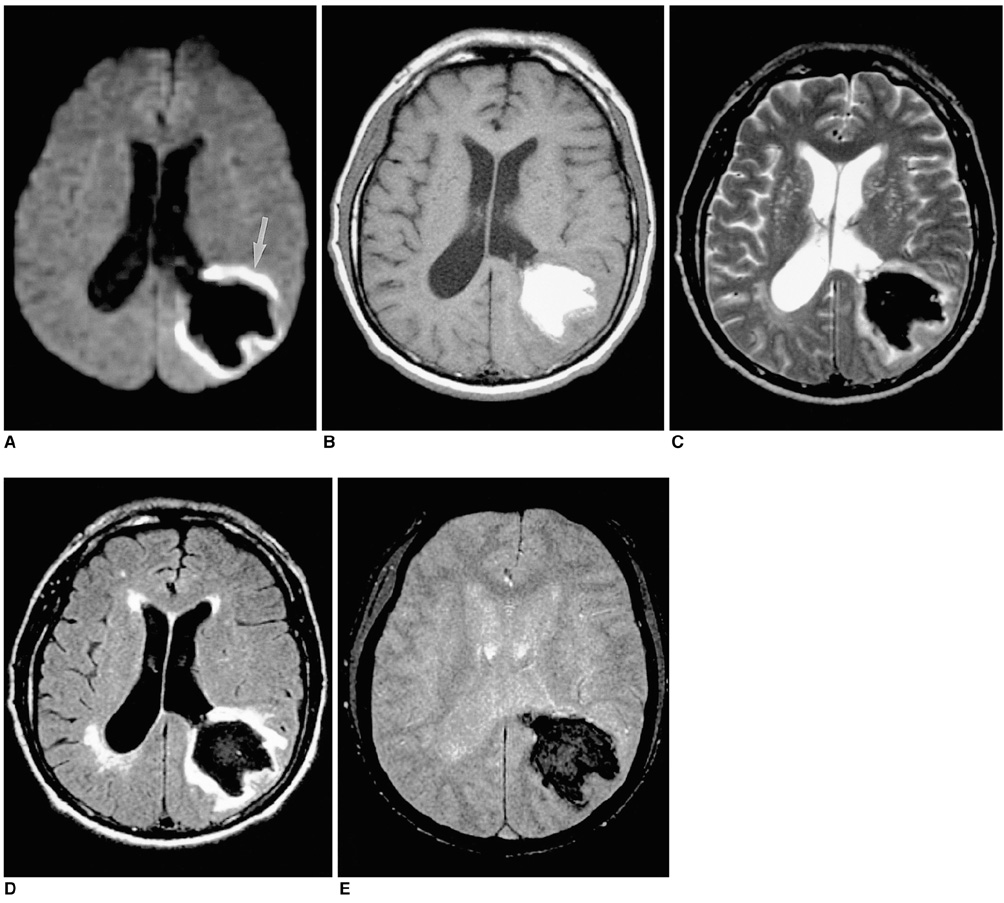
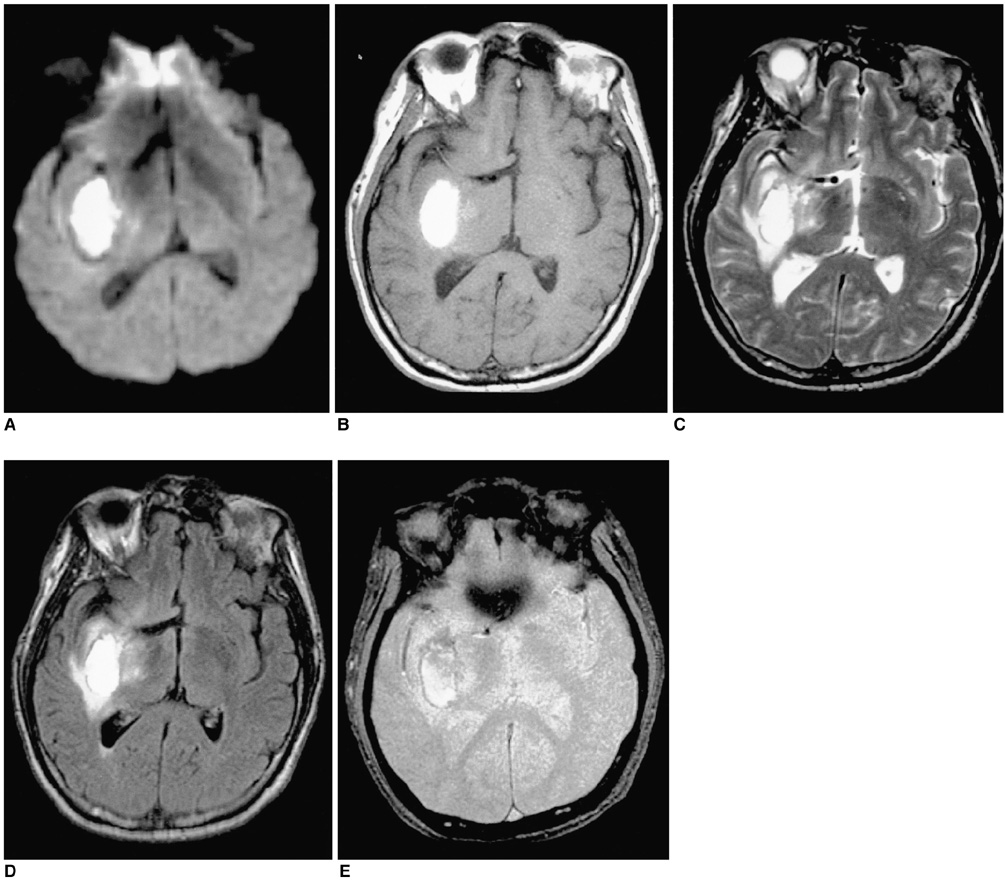
To document the signal characteristics of intracerebral hemorrhage (ICH) at evolving stages on diffusion-weighted images (DWI) by comparison with conventional MR images. MATERIALS AND METHODS: In our retrospective study, 38 patients with ICH underwent a set of imaging sequences that included DWI, T1-and T2-weighted imaging, and fluid-attenuated inversion recovery (FLAIR). In 33 and 10 patients, respectively, conventional and echo-planar T2* gradient-echo images were also obtained. According to the time interval between symptom onset and initial MRI, five stages were categorized: hyperacute (n=6); acute (n=7); early subacute (n=7); late subacute (n=10); and chronic (n=8). We investigated the signal intensity and apparent diffusion coefficient (ADC) of ICH and compared the signal intensities of hematomas at DWI and on conventional MR images. RESULTS: DWI showed that hematomas were hyperintense at the hyperacute and late subacute stages, and hypointense at the acute, early subacute and chronic stages. Invariably, focal hypointensity was observed within a hyperacute hematoma. At the hyperacute, acute and early subacute stages, hyperintense rims that corresponded with edema surrounding the hematoma were present. The mean ADC ratio was 0.73 at the hyperacute stage, 0.72 at the acute stage, 0.70 at the early subacute stage, 0.72 at the late subacute stage, and 2.56 at the chronic stage. CONCLUSION: DWI showed that the signal intensity of an ICH may be related to both its ADC value and the magnetic susceptibility effect. In patients with acute stroke, an understanding of the characteristic features of ICH seen at DWI can be helpful in both the characterization of intracranial hemorrhagic lesions and the differentiation of hemorrhage from ischemia.
MeSH Terms
Figure
Cited by 1 articles
-
Diffusion-Weighted Magnetic Resonance Imaging in the Diagnosis of Cerebral Venous Thrombosis : A Meta-Analysis
Bin Lv, Feng Jing, Cheng-lin Tian, Jian-chao Liu, Jun Wang, Xiang-yu Cao, Xin-feng Liu, Sheng-yuan Yu
J Korean Neurosurg Soc. 2021;64(3):418-426. doi: 10.3340/jkns.2020.0247.
Reference
-
1. Gomori JM, Grossman RI, Goldberg HI, Zimmerman RA, Bilaniuk LT. Intracranial hematomas: imaging by high-field MR. Radiology. 1985. 157:87–93.2. Bradley WG. MR appearance of hemorrhage in the brain. Radiology. 1993. 189:15–26.3. Ebisu T, Tanaka C, Umeda M, et al. Hemorrhagic and nonhemorrhagic stroke: diagnosis with diffusion-weighted and T2-weighted echo-planar MR imaging. Radiology. 1997. 203:823–828.4. Schellinger PD, Jansen O, Fiebach JB, Hacke W, Sartor K. A standardized MRI stroke protocol: comparison with CT in hyperacute intracerebral hemorrhage. Stroke. 1999. 30:765–768.5. Felber S, Auer A, Wolf C, et al. MRI characteristics of spontaneous intracerebral hemorrhage. Radiologe. 1999. 39:838–846.6. Atlas SW, DuBois P, Singer MB, Lu D. Diffusion measurements in intracranial hematomas: implications for MR imaging of acute stroke. Am J Neuroradiol. 2000. 21:1190–1194.7. Carhuapoma JR, Wang PY, Beauchamp NJ, et al. Diffusion-weighted MRI and proton MR spectroscopic imaging in the study of secondary neuronal injury after intracerebral hemorrhage. Stroke. 2000. 31:726–732.8. Wiesmann M, Mayer TE, Yousry I, Hamann GF, Bruckmann H. Detection of hyperacute parenchymal hemorrhage of the brain using echo-planar T2*-weighted and diffusion-weighted MRI. Eur Radiol. 2001. 11:849–853.9. Moseley ME, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990. 14:330–346.10. Chien D, Kwong KK, Gress DR, et al. MR diffusion imaging of cerebral infarction in humans. AJNR. 1992. 13:1097–1102.11. Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996. 199:391–401.12. Marks MP, de Crespigny A, Lentz D, et al. Acute and chronic stroke: navigated spin-echo diffusion-weighted MR imaging. Radiology. 1996. 199:403–408.13. Gonzalez RG, Schaefer P, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999. 210:155–162.14. Sunshine J, Tarr R, Lanzieri C, Landis D, Selman W, Lewin J. Hyperacute stroke: ultrafast MR imaging of triage patients prior to therapy. Radiology. 1999. 212:325–332.15. Patel MR, Edelman RR, Warach S. Detection of hyperacute primary intraparenchymal hemorrhage by magnetic resonance imaging. Stroke. 1996. 27:2321–2324.16. Atlas SW, Thulborn KR. MR detection of hyperacute parenchymal hemorrhage of the brain. AJNR. 1998. 19:1471–1477.17. Linfante I, Llinas RH, Caplan LR, Warach S. MRI features of intracerebral hemorrhage within 2 hours of symptom onset. Stroke. 1999. 30:2263–2267.18. Clark RA, Watanabe AT, Bradley WG, Roberts JD. Acute hematomas: effects of deoxyhemoglobin, hematocrit, and fibrinclot formation and retraction on T2 shortening. Radiology. 1990. 174:201–206.19. Hayman LA, Taber KH, Ford JJ, et al. Effect of clot formation and retraction on spin-echo MR images of blood: an in vitro study. AJNR. 1989. 10:1155–1158.20. Hayman LA, Ford JJ, Taber KH, et al. T2 effect of hemoglobin concentration: assessment with in vitro MR spectroscopy. Radiology. 1988. 168:489–491.21. Beall P, Hazlewood C, Rao P. Nuclear magnetic resonance patterns of intracellular water as a function of HeLa cell cycle. Science. 1976. 192:904–907.22. Hargens A, Bowie L, Lent D, et al. Sickle-cell hemoglobin: fall in osmotic pressure upon deoxygenation. Proc Natl Acad Sci USA. 1980. 77:4310–4312.23. Brooks RA, Di Chiro G, Patronas N. MR imaging of cerebral hematomas at different field strengths: theory and applications. J Comput Assist Tomogr. 1989. 13:194–206.24. Bryant RG, Marill K, Blackmore C, Francis C. Magnetic relaxation in blood and blood clots. Magn Reson Med. 1990. 13:133–144.25. Does MD, Zhong J, Gore JC. In vivo measurement of ADC change due to intravascular susceptibility variation. Magn Reson Med. 1999. 41:236–240.26. Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology. 2000. 217:331–345.27. Thulborn KR, Sorensen AG, Kowall NW, et al. The role of ferritin and hemosiderin in the MR appearance of cerebral hemorrhage: a histopathologic biochemical study in rats. AJNR. 1990. 11:291–297.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion-weighted MR imaging findings of intracerebral hematoma
- A Comparison of Lesion Detection and Conspicuity on T2-weighted Images (T2 FFE), FLAIR and Diffusion-weighted Images in Patients with Traumatic Brain Injury
- The Usefulness of T2* Weighted Magnetic Resonance Image in the Diagnosis of Minute Traumatic Intracerebral Hemorrhage
- Diffusion Weighted MR Image of Intracranial Hemorrhage
- MR Spectroscopy and Diffusion Weighted Imaging Findings of Primary Non-Hodgkin Lymphoma of the Breast: Two Case Reports