Korean J Radiol.
2003 Sep;4(3):170-178. 10.3348/kjr.2003.4.3.170.
MR Evaluation of Radiation Synovectomy of the Knee by Means of Intra-articular Injection of Holmium-166-Chitosan Complex in Patients with Rheumatoid Arthritis: Results at 4-month Follow-up
- Affiliations
-
- 1Department of Diagnostic Radiology, Yonsei University College of Medicine. jss@yumc.yonsei.ac.kr
- 2Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine.
- 3Department of Nuclear Medicine, Yonsei University College of Medicine.
- 4Department of Internal Medicine, Yonsei University College of Medicine.
- KMID: 754021
- DOI: http://doi.org/10.3348/kjr.2003.4.3.170
Abstract
OBJECTIVE
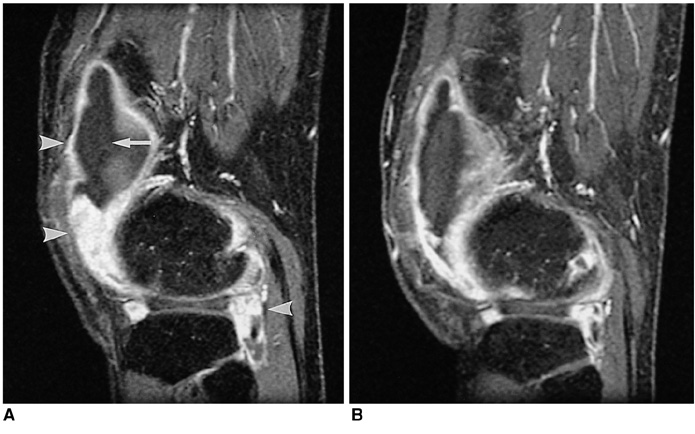
To determine whether MRI is able to demonstrate the effect of radiation synovectomy after the intra-articular injection of holmium-166-chitosan complex for the treatment of rheumatoid arthritis of the knee. MATERIALS AND METHODS: Fourteen patients aged 36-59 years were treated with 10-20 mCi of holmium-166-chitosan complex. A criterion for inclusion in this study was the absence of observable improvement after 3- or more months of treatment of the knee with disease-modifying anti-rheumatic drugs. MR images were acquired both prior to and 4-months after treatment. Clinical evaluation included the use of visual analog scales to assess pain, and the circumference of the knee and its range of motion were also determined. MR evaluation included measurement of the volume of synovial enhancement and wall thickness, the amount of joint effusion, and quantifiable scoring of bone erosion, bone edema and lymph nodes. RESULTS: Visual analog scale readings decreased significantly after radiation synovectomy (p < 0.05). MRI showed that joint effusion decreased significantly (p < 0.05), and that the volume of synovial enhancement tended to decrease, but to an insignificant extent (p = 0.107). CONCLUSION: The decreased joint effusion noted at 4-month follow-up resulted from radiation synovectomy of the rheumatoid knee by means of intra-articular injection of holmium-166-chitosan complex.
Figure
Reference
-
1. Zuckerman JD, Sledge CB, Shortkroff S, Venkatesan P. Treatment of rheumatoid arthritis using radiopharmaceuticals. Int J Rad Appl Instrum B. 1987. 14:211–218.2. Sledge CB, Zuckerman JD, Shortkroff S, et al. Synovectomy of the rheumatoid knee using intra-articular injection of dysprosium-165 ferric hydroxide macroaggregates. J Bone Joint Surg Am. 1987. 69:970–975.3. Sledge CB, Noble J, Hnatowich DJ, Kramer R, Shortkroff S. Experimental radiation synovectomy by 165-Dy ferric hydroxide macroaggregate. Arthritis Rheum. 1977. 20:1334–1342.4. Makela O, Penttila P, Kolehmainen E, Sukura A, Sankari S, Tulamo RM. Experimental radiation synovectomy in rabbit knee with holmium-166 ferric hydroxide macroaggregate. Nucl Med Biol. 2002. 29:593–598.5. Brodack JW, Chinen LK, Deutsch E, Deutsch KF. Studies on the radiolabeling of hydroxyapatite particles for use as radiation synovectomy agents. J Nucl Med. 1992. 33:980.6. Deutsch E, Brodack JW, Deutsch KF. Radiation synovectomy revisited. Eur J Nucl Med. 1993. 20:1113–1127.7. Suzuki YS, Momose Y, Higashi N, et al. Biodistribution and kinetics of Ho-166-chitosan complex in rats and mice. J Nucl Med. 1998. 39:2161–2166.8. Palmer WE, Rosenthal DI, Schoenberg OI, et al. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995. 196:647–655.9. Huh YM, Suh JS, Jeong EK, et al. Role of inflamed synovial volume of the wrist in defining remission of rheumatoid arthritis with gadolinium-enhanced 3D-SPGR imaging. J Magn Reson Imaging. 1999. 10:202–208.10. Ostergaard M, Stoltenberg M, Gideon P, Sorensen K, Henriksen O, Lorenzen I. Changes in synovial membrane and joint effusion volumes after intra-articular methylprednisolone: quantitative assessment of inflammatory and destructive changes in arthritis by MRI. J Rheumatol. 1996. 23:1151–1161.11. Creamer P, Keen M, Zananiri F, et al. Quantitative magnetic resonance imaging of the knee: a method of measuring response to intra-articular treatments. Ann Rheum Dis. 1997. 56:378–381.12. Ostergaard M, Hansen M, Stoltenberg M, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999. 42:918–929.13. Clunie G, Hall-Craggs MA, Paley MN, et al. Measurement of synovial lining volume by magnetic resonance imaging of the knee in chronic synovitis. Ann Rheum Dis. 1997. 56:526–534.14. Alonso-Ruiz A, Perez-Ruiz F, Calabozo M, et al. Efficacy of radiosynovectomy of the knee in rheumatoid arthritis: evaluation with magnetic resonance imaging. Clin Rheumatol. 1998. 17:277–281.15. Konig H, Sieper J, Wolf KJ. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990. 176:473–477.16. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988. 31:315–324.17. Muzzarelli R, Baldassarre V, Conti F, et al. Biological activity of chitosan: ultrastructural study. Biomaterials. 1988. 9:247–252.18. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983. 17:45–56.19. Ohnhaus E, Adlev R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975. 1:379–384.20. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976. 2:175–184.21. Revill SI, Robinson JO, Rosen M, Hogg MI. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976. 31:1191–1198.22. Disler DG, Marr DS, Rosenthal DI. Three-dimensional reconstruction: accuracy of volume measurements of phantoms, with clinical trial. Invest Radiol. 1994. 29:739–745.23. Michael AP, David GD, Thomas RM, et al. Articular cartilage volume in the knee: semiautomated determination from three-dimensional reformations of MR images. Radiology. 1996. 198:855–859.24. Lee JD, Park KK, Lee MG, et al. Radionuclide therapy of skin cancers and Bowen's disease using a specially designed skin patch. J Nucl Med. 1997. 38:697–702.25. Noble J, Jones AG, Davies MA, Sledge CB, Kramer RI, Livni E. Leakage of radioactive particle systems from a synovial joint studied with a gamma camera: its application to radiation synovectomy. J Bone Joint Surg Am. 1983. 65:381–389.26. Lee WY, Moon EY, Lee J, et al. Toxicities of 166Ho-chitosan in mice. Arzneimittelforschung. 1998. 48:300–304.27. Song JS, Suh CH, Park YB, et al. A phase I/IIa study on intra-articular injection of Holmium-166-chitosan complex for the treatment of knee synovitis of rheumatoid arthritis. Eur J Nucl Med. 2001. 28:489–497.28. Johnson LS, Yanch JC, Shortkroff S, Barnes CL, Sitzer AI, Sledge CB. Beta-particle dosimetry in radiation synovectomy. Eur J Nucl Med. 1995. 22:977–988.29. Mumper RJ, Ryo UY, Jay M. Neutron-activated holmium-166-poly (L-lactic acid) microspheres: a potential agent for the internal radiation therapy of hepatic tumors. J Nucl Med. 1991. 32:2139–2143.30. Schumacher HR Jr. Kelley WN, Ruddy S, Harris ED, Sledge CB, editors. Synovial fluid analysis and synovial biopsy. Textbook of rheumatology. 1997. 5th Ed. Philadelphia: Saunders;609–625.31. Harbert JC. Harbert JC, Eckelman WC, Neumann RD, editors. Radionuclide therapy in joint disease. Nuclear medicine: diagnosis and therapy. 1995. New York: Thieme;1093–1109.32. Waterton JC, Ajanayagam V, Ross BD, Brown D, Whittemore A, Johnstone D. Magnetic resonance methods for measurement of disease progression in rheumatoid arthritis. Magn Reson Imaging. 1993. 11:1033–1038.33. Polisson RP, Schoenberg OI, Fischman A, et al. Use of magnetic resonance imaging and positron emission tomography in the assessment of synovial volume and glucose metabolism in patients with rheumatoid arthritis. Arthritis Rheum. 1995. 38:819–825.34. Pirich C, Schwameis E, Bernecker P, et al. Influence of radiation synovectomy on articular cartilage, synovial thickness and enhancement as evidenced by MRI in patients with chronic synovitis. J Nucl Med. 1999. 40:1277–1284.35. Resnick D, Niwayama G. Resnick D, editor. Rheumatoid arthritis and the seronegative spondyloarthropathies: radiographic and pathologic concepts. Diagnosis of bone and joint disorders. 1995. 3rd ed. Philadelphia: Saunders;807–860.36. de Carvalho A, Graudal H, Jorgensen B. Radiologic evaluation of the progression of rheumatoid arthritis. Acta Radiol Diagn (Stockh). 1980. 21:115–121.37. Gubler FM, Maas M, Dijkstra PF, de Jongh HR. Cystic rheumatoid arthritis: description of a nonerosive form. Radiology. 1990. 177:829–834.38. Moore EA, Jacoby RK, Ellis RE, Fry ME, Pittard S, Vennart W. Demonstration of a geode by magnetic resonance imaging: new light on the cause of juxta-articular bone cysts in rheumatoid arthritis. Ann Rheum Dis. 1990. 49:785–787.39. McGonagle D, Gibbon W, O'Connor P, Green M, Pease C, Emery P. Characteristic magnetic resonance imaging entheseal changes of knee synovitis in spondyloarthropathy. Arthritis Rheum. 1998. 41:694–700.40. Lee J, Lee SK, Suh JS, Yoon M, Song JH, Lee CH. Magnetic resonance imaging of the wrist in defining remission of rheumatoid arthritis. J Rheumatol. 1997. 24:1303–1308.41. Kojima M, Hosomura Y, Itoh H, et al. Reactive proliferative lesions in lymph nodes from rheumatoid arthritis patients. a clinicopathological and immunohistological study. Acta Pathol Jpn. 1990. 40:249–254.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preclinical Trial of Radiation Synovectomy with Ho-166
- Radiation Synovectomy by 166Holmium-Chitosan complex in Collagenase Induced Arthritis of the Knee in the Rabbit
- 166Ho - chitosan as a radiation synovectomy agent - Biocompatibility study of 166Ho - chitosan in rabbits
- Synovectomy of the Knee in Rheumatoid Arthritis
- Tuberculous Arthritis of the Knee Associated with Intra - articular Injection of Steroids