Korean J Gastroenterol.
2024 Apr;83(4):150-156. 10.4166/kjg.2024.012.
Comparison of the Efficacy of 12-day Concomitant Quadruple Therapy versus 14-day High dose Dual Therapy as a First-line H. pylori Eradication Regimen
- Affiliations
-
- 1Gut and Liver Research Center, Non-communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
- 2Medicine faculty, Mazandaran University of Medical Sciences, Sari, Iran
- KMID: 2555648
- DOI: http://doi.org/10.4166/kjg.2024.012
Abstract
- Background/Aims
Helicobacter pylori (H. pylori) is the most prevalent infection in the world and is strongly associated with gastric adenocarcinoma, lymphoma and gastric or duodenal ulcers. Different regimens have been used for H. pylori eradication. We aimed to compare the efficacy of two different regimens as first-line H. pylori eradication regimens, in an area with high antibiotic resistance.
Methods
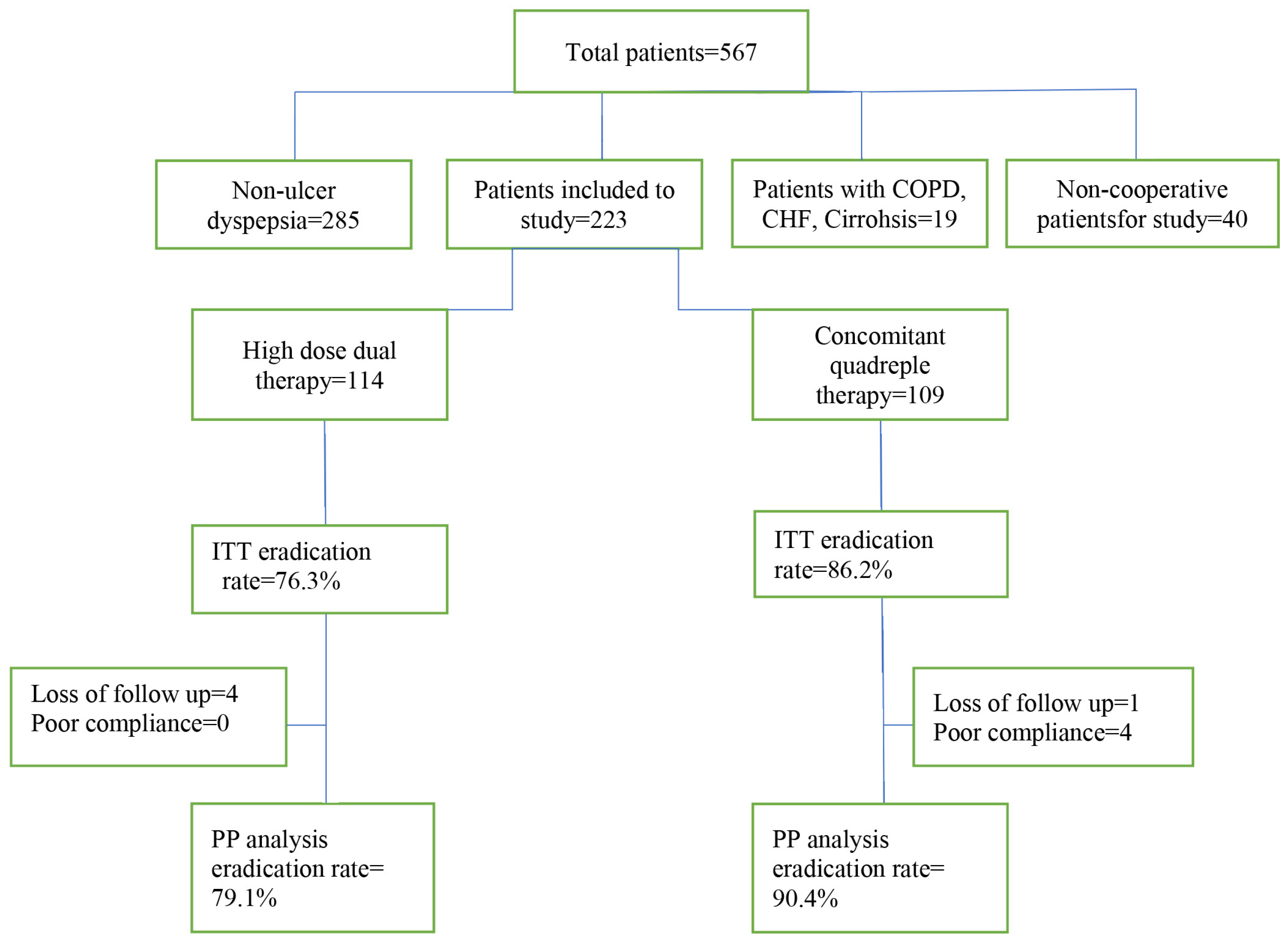
In this RCT, we assigned 223 patients with H. pylori infection, who were naïve to treatment. They were randomly divided into two groups to receive either 12-day concomitant quadruple therapy (consisting of pantoprazole 40 mg, amoxicillin 1 g, clarithromycin 500 mg, and metronidazole 500 mg every 12 hours) or 14-day high dose dual therapy (consisting of esomeprazole 40 mg and amoxicillin 1 g TDS). H. pylori eradication was assessed eight weeks after the end of treatment.
Results
H. pylori eradication rate by PP analysis for 12-day concomitant quadruple therapy and 14-day high dose dual therapy were 90.4% and 79.1%, respectively (p=0.02). According to ITT analysis, the eradication rates were 86.2% and 76.3%, respectively (p=0.06). Adverse drug reactions were 12.3% in high dose dual therapy and 36.8% in concomitant quadruple therapy (p<0.001).
Conclusions
Twelve-day concomitant therapy seems to be an acceptable regimen for first-line H. pylori eradication in Iran, a country with a high rate of antibiotic resistance. Although, high dose dual therapy did not result in an ideal eradication rate, but it had fewer drug side effects than the 12-day concomitant regimen.
Figure
Reference
-
1. Go MF. 2002; Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 16 Suppl 1:3–15. DOI: 10.1046/j.1365-2036.2002.0160s1003.x. PMID: 11849122.2. Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006; (2):CD003840. DOI: 10.1002/14651858.CD003840.pub4.
Article3. Gisbert JP, Calvet X. 2011; Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 34:604–617. DOI: 10.1111/j.1365-2036.2011.04770.x. PMID: 21745241.
Article4. Gisbert JP, Calvet X, O'Connor A, Mégraud F, O'Morain CA. 2010; Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol. 44:313–325. DOI: 10.1097/MCG.0b013e3181c8a1a3. PMID: 20054285.5. Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022; Aug. 8. doi: 10.1136/gutjnl-2022-327745. DOI: 10.1136/gutjnl-2022-327745. PMID: 35944925.6. Fakheri H, Saberi Firoozi M, Bari Z. 2018; Eradication of Helicobacter pylori in Iran: A review. Middle East J Dig Dis. 10:5–17. DOI: 10.15171/mejdd.2017.84. PMID: 29682242. PMCID: PMC5903928.
Article7. Khademi F, Poursina F, Hosseini E, Akbari M, Safaei HG. 2015; Helicobacter pylori in Iran: A systematic review on the antibiotic resistance. Iran J Basic Med Sci. 18:2–7.8. Saniee P, Hosseini F, Kadkhodaei S, Siavoshi F, Khalili-Samani S. 2018; Helicobacter pylori multidrug resistance due to misuse of antibiotics in Iran. Arch Iran Med. 21:283–288.9. Zullo A, Scaccianoce G, De Francesco V, et al. 2013; Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol. 37:647–650. DOI: 10.1016/j.clinre.2013.04.003. PMID: 23747131.
Article10. Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. 2013; Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 145:121–128.e1. DOI: 10.1053/j.gastro.2013.03.050. PMID: 23562754.
Article11. Park SM, Kim JS, Kim BW, Ji JS, Choi H. 2017; Randomized clinical trial comparing 10- or 14-day sequential therapy and 10- or 14-day concomitant therapy for the first line empirical treatment of Helicobacter pylori infection. J Gastroenterol Hepatol. 32:589–594. DOI: 10.1111/jgh.13510. PMID: 27505301.
Article12. De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. 2014; Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol. 63:748–752. DOI: 10.1099/jmm.0.072322-0. PMID: 24586031.
Article13. Choe JW, Jung SW, Kim SY, et al. 2018; Comparative study of Helicobacter pylori eradication rates of concomitant therapy vs modified quadruple therapy comprising proton-pump inhibitor, bismuth, amoxicillin, and metronidazole in Korea. Helicobacter. 23:e12466. DOI: 10.1111/hel.12466. PMID: 29369454.
Article14. Alhooei S, Tirgar Fakheri H, Hosseini V, et al. 2016; A Comparison between hybrid and concomitant regimens for Helicobacter pylori eradication: A randomized clinical trial. Middle East J Dig Dis. 8:219–225. DOI: 10.15171/mejdd.2016.24. PMID: 27698972. PMCID: PMC5045675.
Article15. Bari Z, Fakheri H, Taghvaei T, Yaghoobi M. 2020; A Comparison between 10-day and 12-day concomitant regimens for Helicobacter pylori eradication: A randomized clinical trial. Middle East J Dig Dis. 12:106–110. DOI: 10.34172/mejdd.2020.169. PMID: 32626563. PMCID: PMC7320987.
Article16. Yao CC, Kuo CM, Hsu CN, et al. 2019; First-line Helicobacter pylori eradication rates are significantly lower in patients with than those without type 2 diabetes mellitus. Infect Drug Resist. 12:1425–1431. DOI: 10.2147/IDR.S194584. PMID: 31239721. PMCID: PMC6554512.17. Zullo A, Ridola L, Francesco VD, et al. 2015; High-dose esomeprazole and amoxicillin dual therapy for first-line Helicobacter pylori eradication: a proof of concept study. Ann Gastroenterol. 28:448–451.18. Yang X, Wang JX, Han SX, Gao CP. 2019; High dose dual therapy versus bismuth quadruple therapy for Helicobacter pylori eradication treatment: A systematic review and meta-analysis. Medicine (Baltimore). 98:e14396. DOI: 10.1097/MD.0000000000014396. PMID: 30762742. PMCID: PMC6408008.19. Yang J, Zhang Y, Fan L, et al. 2019; Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol. 114:437–445. DOI: 10.14309/ajg.0000000000000132. PMID: 30807294.
Article20. Yang JC, Lin CJ, Wang HL, et al. 2015; High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 13:895–905.e5. DOI: 10.1016/j.cgh.2014.10.036. PMID: 25460556. PMCID: PMC4404168.21. Yu L, Luo L, Long X, et al. 2019; High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: A randomized trial. Helicobacter. 24:e12596. DOI: 10.1111/hel.12596. PMID: 31111580.
Article22. Yadollahi B, Valizadeh Toosi SM, Bari Z, et al. 2022; Efficacy of 14-day concomitant quadruple therapy and 14-day high-dose dual therapy on H. pylori eradication. Gastroenterol Hepatol Bed Bench. 15:172–178.23. Cheng A, Sheng WH, Liou JM, et al. 2015; Comparative in vitro antimicrobial susceptibility and synergistic activity of antimicrobial combinations against Helicobacter pylori isolates in Taiwan. J Microbiol Immunol Infect. 48:72–79. DOI: 10.1016/j.jmii.2012.08.021. PMID: 23036269.
Article24. Salcedo JA, Al-Kawas F. 1998; Treatment of Helicobacter pylori infection. Arch Intern Med. 158:842–851. DOI: 10.1001/archinte.158.8.842. PMID: 9570169.
Article25. Graham DY, Lu H, Yamaoka Y. 2007; A report card to grade Helicobacter pylori therapy. Helicobacter. 12:275–278. DOI: 10.1111/j.1523-5378.2007.00518.x. PMID: 17669098.26. Graham DY, Lee YC, Wu MS. 2014; Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 12:177–186.e3. DOI: 10.1016/j.cgh.2013.05.028. PMID: 23751282. PMCID: PMC3830667.
Article27. Mohammadi M, Doroud D, Mohajerani N, Massarrat S. 2005; Helicobacter pylori antibiotic resistance in Iran. World J Gastroenterol. 11:6009–6013. DOI: 10.3748/wjg.v11.i38.6009. PMID: 16273615. PMCID: PMC4436725.28. Farzi N, Yadegar A, Sadeghi A, et al. 2019; High prevalence of antibiotic resistance in Iranian Helicobacter pylori Isolates: Importance of functional and mutational analysis of resistance genes and virulence genotyping. J Clin Med. 8:2004. DOI: 10.3390/jcm8112004. PMID: 31744181. PMCID: PMC6912791.
Article29. Hu JL, Yang J, Zhou YB, Li P, Han R, Fang DC. 2017; Optimized high-dose amoxicillin-proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J Gastroenterol. 23:275–280. DOI: 10.4103/sjg.SJG_91_17. PMID: 28937021. PMCID: PMC5625363.
Article30. Zhang Y, Zhu YJ, Zhao Z, et al. 2020; Efficacy of modified esomeprazole-amoxicillin dual therapies for Helicobacter pylori infection: an open-label, randomized trial. Eur J Gastroenterol Hepatol. 32:563–568. DOI: 10.1097/MEG.0000000000001646. PMID: 31851093.
Article31. Yuan Y, Ford AC, Khan KJ, et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst Rev. 2013; (12):CD008337. DOI: 10.1002/14651858.CD008337.pub2. PMCID: PMC10114080.
Article32. Ierardi E, Losurdo G, Fortezza RF, Principi M, Barone M, Leo AD. 2019; Optimizing proton pump inhibitors in Helicobacter pylori treatment: Old and new tricks to improve effectiveness. World J Gastroenterol. 25:5097–5104. DOI: 10.3748/wjg.v25.i34.5097. PMID: 31558859. PMCID: PMC6747288.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ten-day tegoprazan-based concomitant therapy as a first-line treatment for Helicobacter pylori eradication
- Recent First Line Eradication Rate of Helicobacter pylori Infection: Single Center Experience
- First-line Helicobacter pylori Eradication with Standard Triple Therapy and Concomitant Therapy: A Retrospective Study
- Ten-day Sequential Therapy versus Bismuth Based Quadruple Therapy as Second Line Treatment for Helicobacter pylori Infection
- Effect of 7-day Bismuth Quadruple Therapy versus 14-day Moxifloxacin Triple Therapy for Second-line Helicobacter pylori Eradication Therapy