Intest Res.
2024 Apr;22(2):199-207. 10.5217/ir.2023.00162.
Unraveling molecular similarities between colorectal polyps and colorectal cancer: a systems biology approach
- Affiliations
-
- 1Department of Biology, School of Sciences, Razi University, Kermanshah, Islamic Republic of Iran
- KMID: 2554663
- DOI: http://doi.org/10.5217/ir.2023.00162
Abstract
- Background/Aims
Colorectal cancer (CRC) and colorectal polyps are intimately linked, with polyps acting as precursors to CRC. Understanding the molecular mechanisms governing their development is crucial for advancing diagnosis and treatment. Employing a systems biology approach, we investigated the molecular similarities between polyp and CRC.
Methods
We analyzed gene expression profiles, protein-protein interactions, transcription factors, and gene ontology to identify common differentially expressed genes (DEGs) and unravel shared molecular pathways.
Results
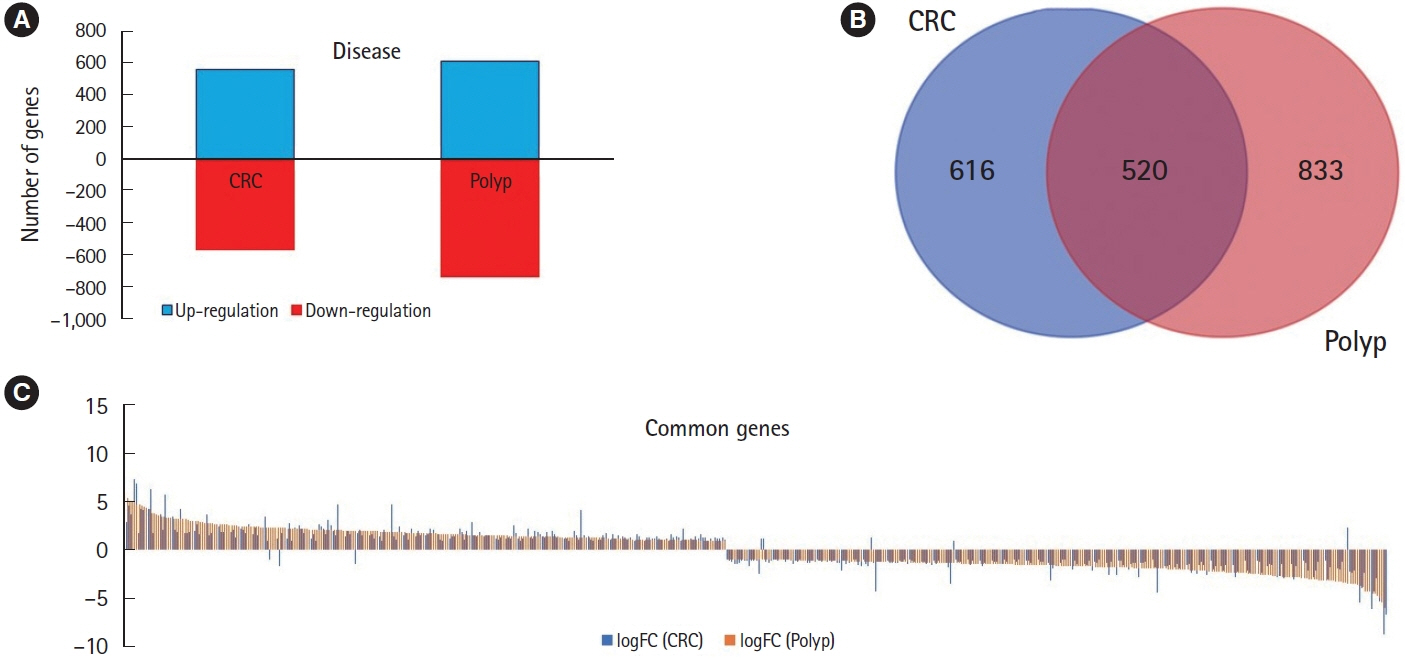
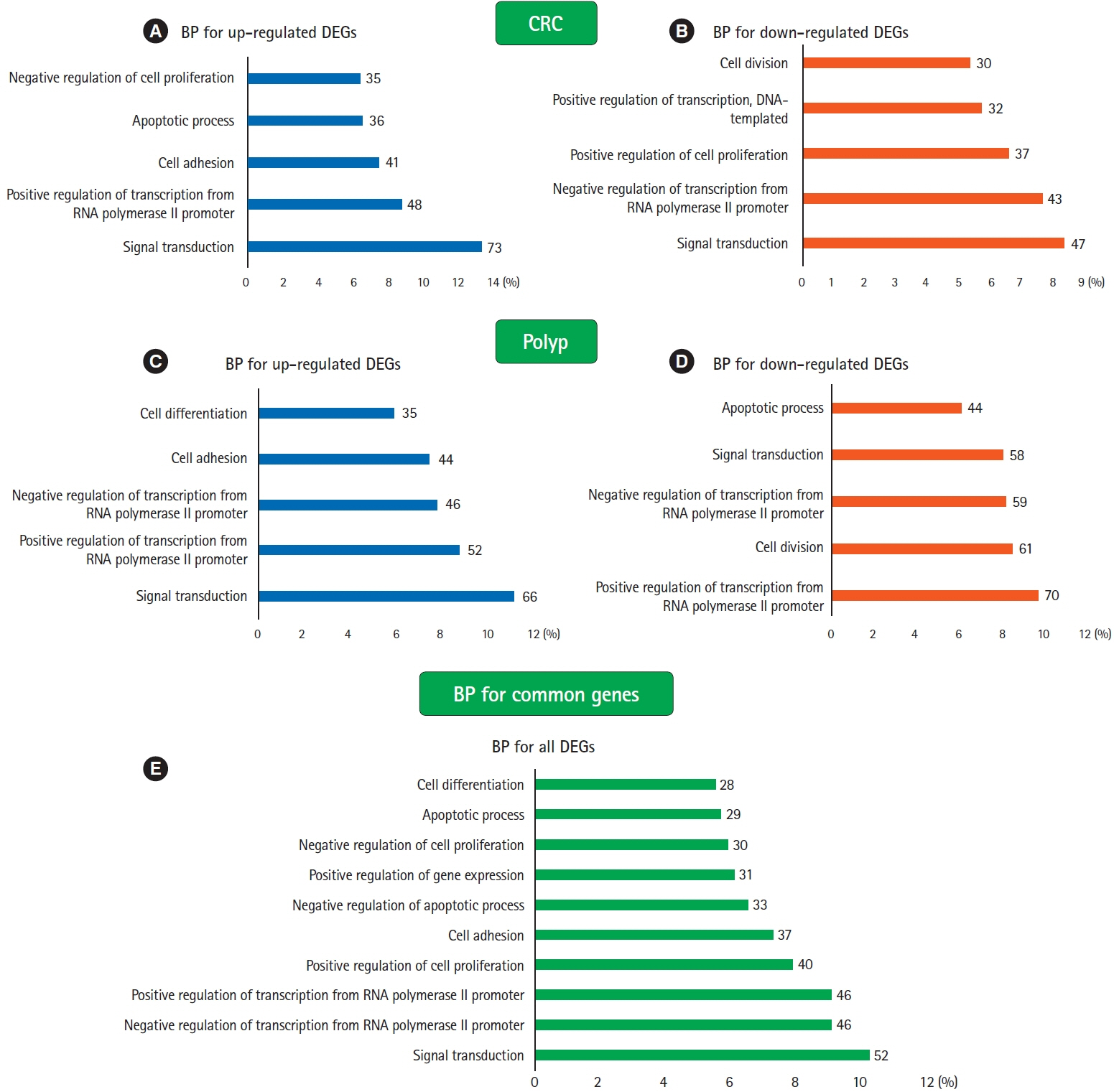
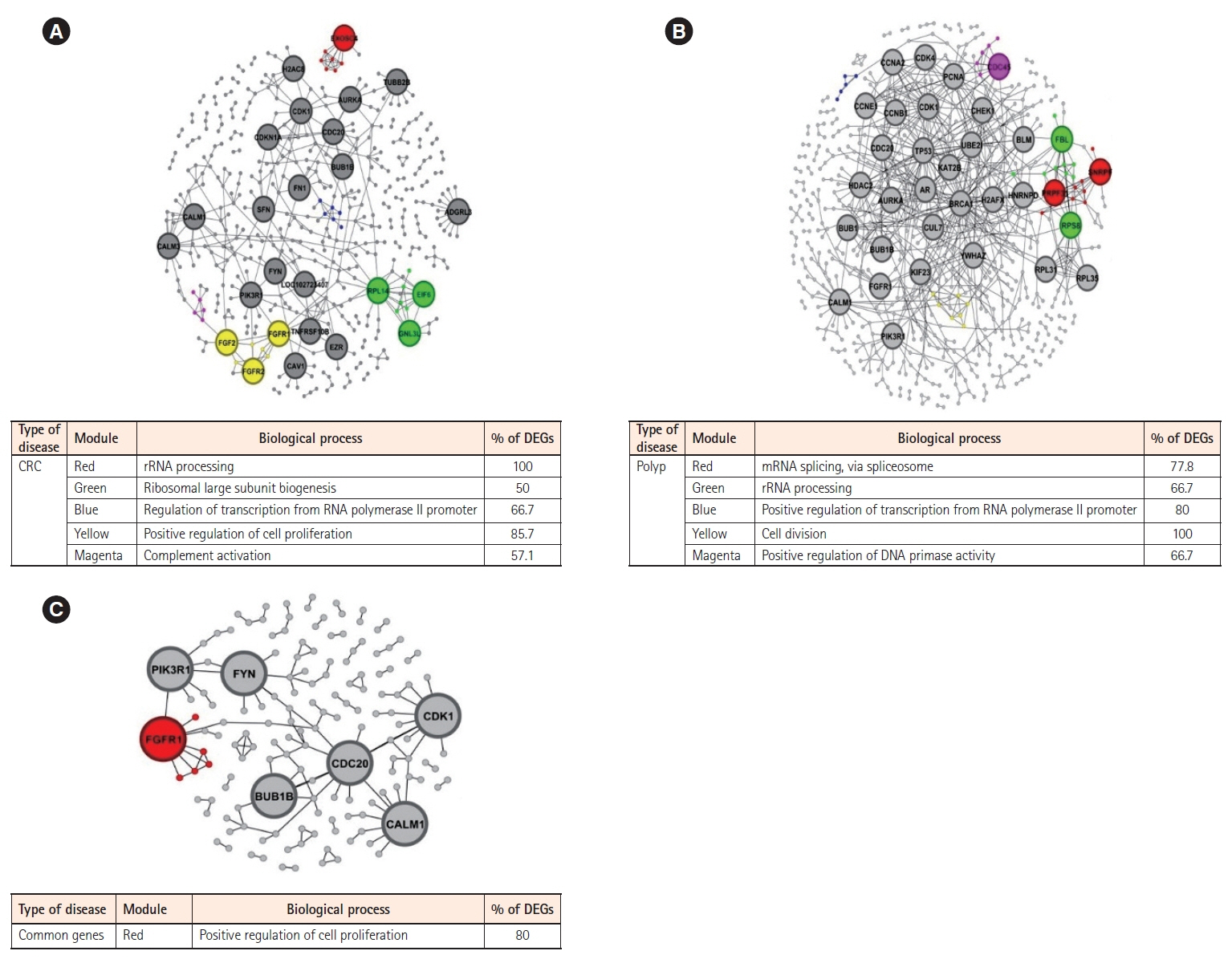
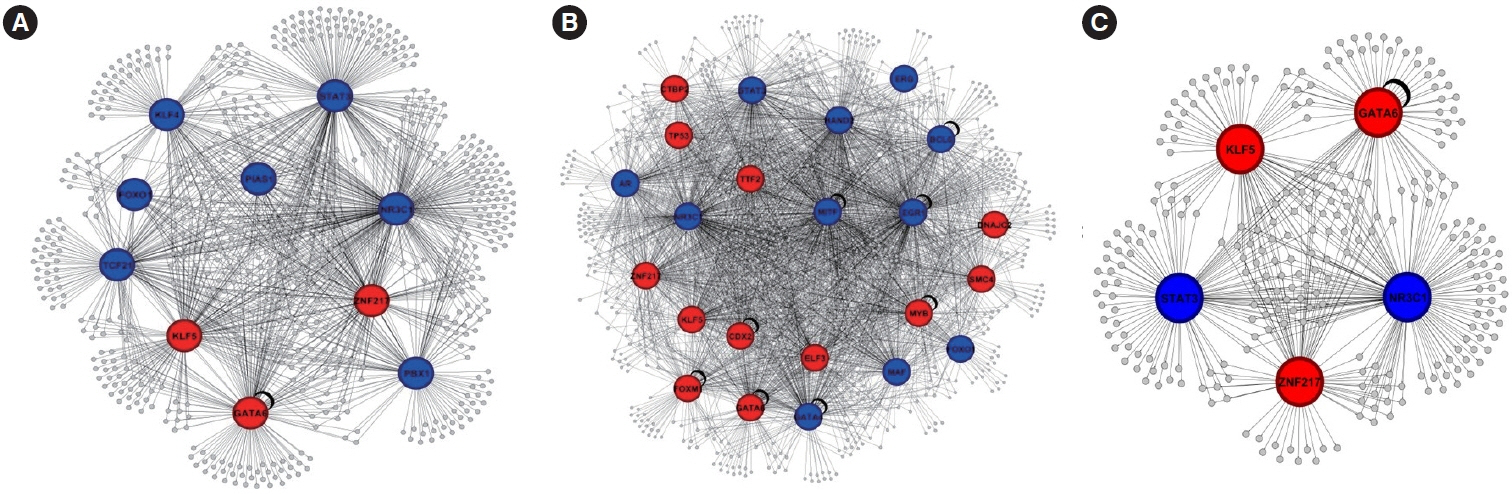
Our analysis revealed 520 commonly dysregulated genes in polyps and CRC, serving as potential biomarkers and pivotal contributors to disease progression. Gene ontology analysis elucidated distinct biological processes associated with upregulated and downregulated DEGs in both conditions, highlighting common pathways, including signal transduction, cell adhesion, and positive regulation of cell proliferation. Moreover, protein-protein interaction networks shed light on subnetworks involved in rRNA processing, positive regulation of cell proliferation, mRNA splicing, and cell division. Transcription factor analysis identified major regulators and differentially expressed transcription factors in polyp and CRC. Notably, we identified common differentially expressed transcription factors, including ZNF217, NR3C1, KLF5, GATA6, and STAT3, with STAT3 and NR3C1 exhibiting increased expression.
Conclusions
This comprehensive analysis enriches our understanding of the molecular mechanisms underlying polyp formation and CRC development, providing potential targets for further investigation and therapeutic intervention. Our findings contribute substantively to crafting personalized strategies for refining the diagnosis and treatment of polyps and CRC.
Keyword
Figure
Reference
-
1. Bozic D, Baralić K, Živančević K, et al. Predicting sulforaphaneinduced adverse effects in colon cancer patients via in silico investigation. Biomed Pharmacother. 2022; 146:112598.
Article2. Alzahrani SM, Al Doghaither HA, Al-Ghafari AB. General insight into cancer: an overview of colorectal cancer (Review). Mol Clin Oncol. 2021; 15:271.
Article3. Godkhindi AM, Gowda RM. Automated detection of polyps in CT colonography images using deep learning algorithms in colon cancer diagnosis. Proceedings of the 2017 International Conference on Energy, Communication, Data Analytics and Soft Computing (ICECDS); 2017 Aug 1-2; Chennai. IEEE; 2017. p. 1722-1728.4. Tanwar S, Goel PM, Johri P, Divan MJ. Classification of benign and malignant colorectal polyps using pit pattern classification. Proceedings of the 4th International Conference: Innovative Advancement in Engineering & Technology (IAET); 2020 Feb 21-22; Jaipur. SSRN; 2020. https://doi.org/10.2139/ssrn.3558374.5. Suárez J, Mata E, Guerra A, et al. Impact of the COVID-19 pandemic during Spain’s state of emergency on the diagnosis of colorectal cancer. J Surg Oncol. 2021; 123:32–36.
Article6. González LV, de Miguel Ibáñez R, Sotos FE. Colorectal cancer prevalence and survival in Cuenca (Spain). J Gastrointest Cancer. 2023; 54:80–89.
Article7. Niu Y, Xue J, Wu X, et al. Clinical significance of serum haptoglobin and protein disulfide-isomerase A3 in the screening, diagnosis, and staging of colorectal cancer. Front Pharmacol. 2022; 13:935500.
Article8. Chai X, Li Y, Yin Z, et al. Association of meat subtypes with colorectal polyp prevalence: finding from the Lanxi Pre-colorectal Cancer Cohort in China. Front Nutr. 2022; 9:833571.
Article9. Battaglin F, Naseem M, Lenz HJ, Salem ME. Microsatellite instability in colorectal cancer: overview of its clinical significance and novel perspectives. Clin Adv Hematol Oncol. 2018; 16:735–745.10. Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: diagnosis, prognosis and treatment. Eur J Cancer. 2022; 175:136–157.
Article11. Wimmer K, Etzler J. Constitutional mismatch repair-deficiency syndrome: have we so far seen only the tip of an iceberg? Hum Genet. 2008; 124:105–122.
Article12. Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014; 25:1032–1038.
Article13. Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017; 45–D362-D368.
Article14. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13:2498–2504.
Article15. Bastian M, Heymann S, Jacomy M. Gephi: an open source software for exploring and manipulating networks. Proceedings of the Third International AAAI Conference on Web and Social Media; 2009 May 17-20; San Jose, CA. AAAI Press; 2009. p. 361-362.16. Nepusz T, Yu H, Paccanaro A. Detecting overlapping protein complexes in protein-protein interaction networks. Nat Methods. 2012; 9:471–472.
Article17. Lachmann A, Xu H, Krishnan J, Berger SI, Mazloom AR, Ma’ayan A. ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics. 2010; 26:2438–2444.
Article18. Huang DW, Sherman BT, Zheng X, et al. Extracting biological meaning from large gene lists with DAVID. Curr Protoc Bioinformatics. 2009; 27:13.11.1–13.11.13.
Article19. Song M, Emilsson L, Roelstraete B, Ludvigsson JF. Risk of colorectal cancer in first degree relatives of patients with colorectal polyps: nationwide case-control study in Sweden. BMJ. 2021; 373–n877.
Article20. Wang SS, Zhai GQ, Huang ZG, et al. Nitidine chloride regulates cell function of bladder cancer in vitro through downregulating lymphocyte antigen 75. Naunyn Schmiedebergs Arch Pharmacol. 2023; 396:2071–2085.
Article21. Yang K, Shen J, Tan FQ, Zheng XY, Xie LP. Antitumor activity of small activating RNAs induced PAWR gene activation in human bladder cancer cells. Int J Med Sci. 2021; 18:3039–3049.
Article22. Zhang W, Zhu J, He X, et al. Exosome complex genes mediate RNA degradation and predict survival in mantle cell lymphoma. Oncol Lett. 2019; 18:5119–5128.
Article23. Mouterde M, Daali Y, Rollason V, et al. Joint analysis of phenotypic and genomic diversity sheds light on the evolution of xenobiotic metabolism in humans. Genome Biol Evol. 2022; 14:evac167.
Article24. Dai L, Mugaanyi J, Zhang T, et al. A pan-cancer bioinformatic analysis of the carcinogenic role of SMARCA1 in human carcinomas. PLoS One. 2022; 17:e0274823.
Article25. Brien GL, Stegmaier K, Armstrong SA. Targeting chromatin complexes in fusion protein-driven malignancies. Nat Rev Cancer. 2019; 19:255–269.
Article26. Chen X, Xu W, Ma Z, et al. Circ_0000215 exerts oncogenic function in nasopharyngeal carcinoma by targeting miR-512-5p. Front Cell Dev Biol. 2021; 9:688873.
Article27. Wu F, Sun Y, Chen J, et al. The oncogenic role of APC/C activator protein Cdc20 by an integrated pan-cancer analysis in human tumors. Front Oncol. 2021; 11:721797.
Article28. Uehara R, Yamada E, Uehara M, et al. Tgm2-p62-p53 complex may function as an autophagic regulator through Fyn and involve in diabetic kidney disease. J Endocr Soc. 2021; 5(Suppl 1):A442–A443.
Article29. Chew NJ, Nguyen EV, Su SP, et al. FGFR3 signaling and function in triple negative breast cancer. Cell Commun Signal. 2020; 18:13.
Article30. Li X, Wang W, Ding X. Pan-cancer investigation of psoriasisrelated BUB1B gene: genetical alteration and oncogenic immunology. Sci Rep. 2023; 13:6058.
Article31. Hydbring P, Malumbres M, Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat Rev Mol Cell Biol. 2016; 17:280–292.
Article32. Alcalde J, Munk M, González-Muñoz M, Panina S, Berchtold MW, Villalobo A. Calmodulin downregulation in conditional knockout HeLa cells inhibits cell migration. Arch Biochem Biophys. 2021; 697:108680.
Article33. Sun G, Qin J, Qiu Y, et al. Microarray analysis of gene expression in the ovarian cancer cell line HO-8910 with silencing of the ZNF217 gene. Mol Med Rep. 2009; 2:851–855.
Article34. Chia NY, Deng N, Das K, et al. Regulatory crosstalk between lineage-survival oncogenes KLF5, GATA4 and GATA6 cooperatively promotes gastric cancer development. Gut. 2015; 64:707–719.
Article35. Sun J, Zhou H, Bao X, et al. lncRNA TUG1 facilitates colorectal cancer stem cell characteristics and chemoresistance by enhancing GATA6 protein stability. Stem Cells Int. 2021; 2021:1075481.
Article36. Li YY. The effect of a small interfering RNA targeting STAT3 gene on the biological function of human endometrial cancer cell line HEC-1B. Tumor. 2013; (12):1054–1060.37. Chen W, Zhang J, Fu H, et al. KLF5 is activated by gene amplification in gastric cancer and is essential for gastric cell proliferation. Cells. 2021; 10:1002.
Article38. Kim DJ, Chan KS, Sano S, Digiovanni J. Signal transducer and activator of transcription 3 (Stat3) in epithelial carcinogenesis. Mol Carcinog. 2007; 46:725–731.
Article39. Park YY. Genomic analysis of nuclear receptors and miRNAs identifies a role for the NR3C1/miR-200 axis in colon cancer. Genes Genomics. 2021; 43:913–920.
Article40. Xiong H, Chen Z, Lin B, et al. Naringenin regulates FKBP4/NR3C1/NRF2 axis in autophagy and proliferation of breast cancer and differentiation and maturation of dendritic cell. Front Immunol. 2022; 12:745111.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resection of Diminutive and Small Colorectal Polyps: What Is the Optimal Technique?
- Screening strategy for colorectal cancer according to risk
- Crossroad between inflammation and carcinogenesis in colon
- Detection of Polyps After Resection of Colorectal Cancer
- Colorectal Polyps : Endoscopic Diagnosis and Polypectomy