J Rheum Dis.
2024 Apr;31(2):79-85. 10.4078/jrd.2023.0043.
Effect of recombinant human bone morphogenetic protein-2 and osteoprotegerin-Fc in MC3T3-E1 cells
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- 2Department of Emergency Medical Technology, Gyeongbuk Provincial College, Yecheon, Korea
- 3Department of Biomedical Engineering, Korea University College of Health Science, Seoul, Korea
- 4Department of Rheumatology, Eulji Rheumatology Research Institute, Eulji University School of Medicine, Uijeongbu, Korea
- KMID: 2554323
- DOI: http://doi.org/10.4078/jrd.2023.0043
Abstract
Objective
We compared the osteoblastogenesis by serially administrating recombinant human bone morphogenetic protein-2 (rhBMP-2) and osteoprotegerin-immunoglobulin Fc segment complex (OPG-Fc).
Methods
The MC3T3-E1 preosteoblast cell line was differentiated for 1, 3, and 7 days with a treatment of OPG-Fc in 10~200 ng/mL concentration and the cell viability was evaluated by Cell Counting Kit-8 analysis. The level of differentiation from MC3T3-E1 cells to osteoblasts was determined by alkaline phosphatase activity. The level of runt domain-containing transcription factor 2 (Runx2) and osteopontin (OPN) manifestation, involved in osteoblast differentiation, was examined by real-time polymerase chain reaction and western blotting.
Results
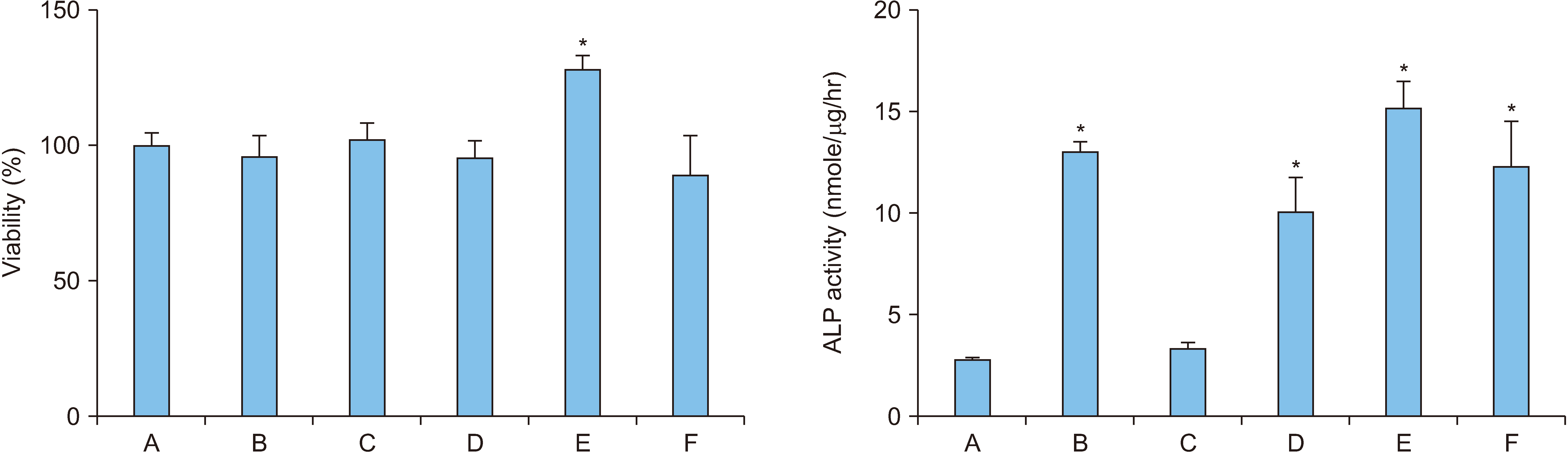
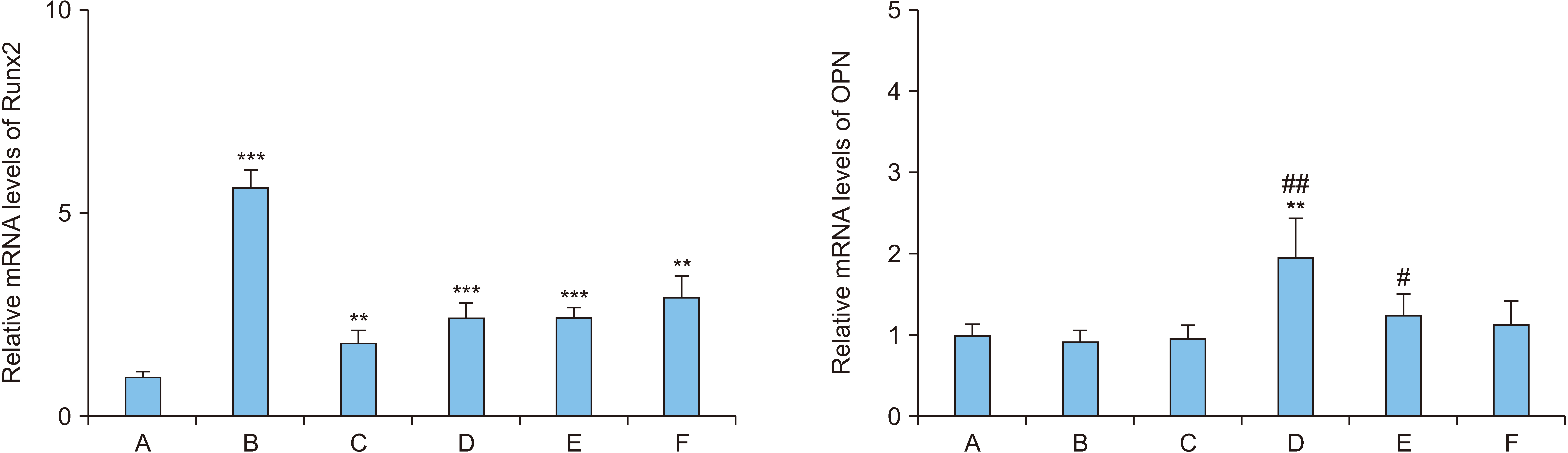
During MC3T3-E1 cell differentiation, the differentiation level was high with 1-day treatment using 100 ng/mL OPGFc. The treatment with 50 ng/mL rhBMP-2 for 7 days, followed by 1-day treatment with 100 ng/mL OPG-Fc produced the highest differentiation level, which was approximately 5.3 times that of the control group (p<0.05). The expression of Runx2 mRNA significantly increased, reaching 2.5 times the level of the control group under the condition of 7-day treatment with rhBMP-2 and 1-day treatment with OPG-Fc (p<0.001). The expression of Runx2 protein significantly increased to approximately 5.7 times that of the control group under the condition of 7-day treatment with rhBMP-2, followed by 1-day treatment with OPG-Fc (p<0.01). The expression of OPN protein showed no change from that of the control group under various conditions of rhBMP-2 and OPGFc combinations.
Conclusion
These results imply that the treating preosteoblasts with rhBMP-2 first and then with OPG-Fc increased osteoblast differentiation efficacy.
Keyword
Figure
Reference
-
1. Amarasekara DS, Kim S, Rho J. 2021; Regulation of osteoblast differentiation by cytokine networks. Int J Mol Sci. 22:2851. DOI: 10.3390/ijms22062851. PMID: 33799644. PMCID: PMC7998677.
Article2. Quinlan E, Thompson EM, Matsiko A, O'Brien FJ, López-Noriega A. 2015; Long-term controlled delivery of rhBMP-2 from collagen-hydroxyapatite scaffolds for superior bone tissue regeneration. J Control Release. 207:112–9. DOI: 10.1016/j.jconrel.2015.03.028. PMID: 25817394.
Article3. Lackington WA, Gehweiler D, Zhao E, Zderic I, Nehrbass D, Zeiter S, et al. 2022; Interleukin-1 receptor antagonist enhances the therapeutic efficacy of a low dose of rhBMP-2 in a weight-bearing rat femoral defect model. Acta Biomater. 149:189–97. DOI: 10.1016/j.actbio.2022.07.012. PMID: 35840106.
Article4. Tateiwa D, Kaito T, Hashimoto K, Okada R, Kodama J, Kushioka J, et al. 2022; Selective retinoic acid receptor γ antagonist 7C is a potent enhancer of BMP-induced ectopic endochondral bone formation. Front Cell Dev Biol. 10:802699. DOI: 10.3389/fcell.2022.802699. PMID: 35359440. PMCID: PMC8963923.
Article5. Aspenberg P, Agholme F, Magnusson P, Fahlgren A. 2011; Targeting RANKL for reduction of bone loss around unstable implants: OPG-Fc compared to alendronate in a model for mechanically induced loosening. Bone. 48:225–30. DOI: 10.1016/j.bone.2010.09.024. PMID: 20858557.
Article6. Huang P, Wang Y, Chi ZY, Yang ZY, Ni J, Yang WJ, et al. 2005; [In vitro study of combination rhOPG-Fc and alendronate on inhibition osteoclast]. Zhonghua Wai Ke Za Zhi. 43:812–6. Chinese.7. Wang Y, Huang P, Tang PF, Chan KM, Li G. 2011; Alendronate (ALN) combined with osteoprotegerin (OPG) significantly improves mechanical properties of long bone than the single use of ALN or OPG in the ovariectomized rats. J Orthop Surg Res. 6:34. DOI: 10.1186/1749-799X-6-34. PMID: 21752290. PMCID: PMC3143091.
Article8. Kim SH, Choi HJ, Yoon DS, Son CN. 2021; Serial administration of rhBMP-2 and alendronate enhances the differentiation of osteoblasts. Int J Rheum Dis. 24:1266–72. DOI: 10.1111/1756-185X.14189. PMID: 34324274.
Article9. Zhang Q, Chen S, Shi J, Li F, Shi X, Hu X, et al. 2019; Coupled OPG-Fc on decellularized aortic valves by EDC/NHS attenuates rat MSCs calcification in vitro. ASAIO J. 65:197–204. DOI: 10.1097/MAT.0000000000000796. PMID: 29677036.
Article10. Yu H, de Vos P, Ren Y. 2011; Overexpression of osteoprotegerin promotes preosteoblast differentiation to mature osteoblasts. Angle Orthod. 81:100–6. DOI: 10.2319/050210-238.1. PMID: 20936961. PMCID: PMC8926369.
Article11. Lee D, Wufuer M, Kim I, Choi TH, Kim BJ, Jung HG, et al. 2021; Sequential dual-drug delivery of BMP-2 and alendronate from hydroxyapatite-collagen scaffolds for enhanced bone regeneration. Sci Rep. 11:746. DOI: 10.1038/s41598-020-80608-3. PMID: 33436904. PMCID: PMC7804460.
Article12. Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH, et al. 2016; Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone. 84:93–103. DOI: 10.1016/j.bone.2015.12.052. PMID: 26723577. PMCID: PMC4903101.
Article13. Ikebuchi Y, Aoki S, Honma M, Hayashi M, Sugamori Y, Khan M, et al. 2018; Coupling of bone resorption and formation by RANKL reverse signalling. Nature. 561:195–200. DOI: 10.1038/s41586-018-0482-7. PMID: 30185903.
Article14. Liu Y, Lin D, Li B, Hong H, Jiang C, Yuan Y, et al. 2022; BMP-2/CPC scaffold with dexamethasone-loaded blood clot embedment accelerates clinical bone regeneration. Am J Transl Res. 14:2874–93.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone Morphogenetic Protein-2 Desensitizes MC3T3-E1 Osteoblastic Cells to Estrogen Through Transcriptional Downregulation of Estrogen Receptor 1
- Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells
- Icaritin, a Flavonoid Derived from the Herb Epimedium, Promotes Osteogenic Differentiation of MC3T3-E1 Cells
- On the Activity of MC3T3-E1 Cell in vitro
- Combined effect of recombinant human bone morphogenetic protein-2 and low level laser irradiation on bisphosphonate-treated osteoblasts