Nutr Res Pract.
2016 Apr;10(2):148-153. 10.4162/nrp.2016.10.2.148.
Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells
- Affiliations
-
- 1Department of Food and Nutrition, College of Medical and Life Science, Silla University, 140 Baegyang-daero, 700beon-gil, Sasang-Gu, Busan 46958, Korea. mihkim@silla.ac.kr
- 2Department of Pharmaceutical Engineering, College of Medical and Life Science, Silla University, Busan 46958, Korea.
- 3ISFOOD Co. LTD., 7, Hoenggye-gil, Ilgwang-myeon, Gijang-gun, Busan 46048, Korea.
- KMID: 2313911
- DOI: http://doi.org/10.4162/nrp.2016.10.2.148
Abstract
- BACKGROUND/OBJECTIVES
Bone formation and bone resorption continuously occur in bone tissue to prevent the accumulation of old bone, this being called bone remodeling. Osteoblasts especially play a crucial role in bone formation through the differentiation and proliferation. Therefore, in this study, we investigated the effects of Scytosiphon lomentaria extract (SLE) on osteoblastic proliferation and differentiation in MC3T3-E1 cells.
MATERIALS/METHODS
A cell proliferation assay, alkaline phosphatase (ALP) activity assay, alizarin red staining and protein expression analysis of osteoblastic genes were carried out to assess the osteoblastic proliferation and differentiation.
RESULTS
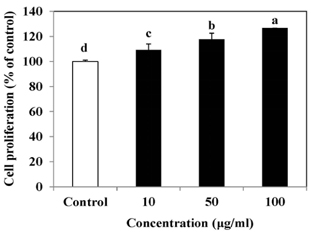
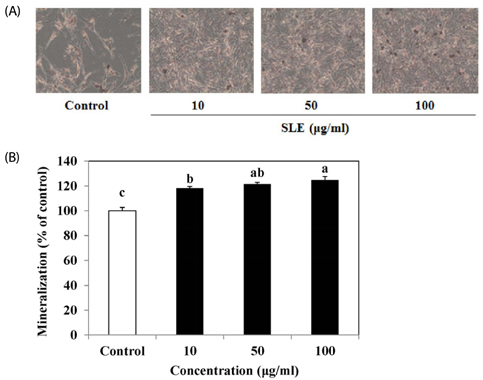
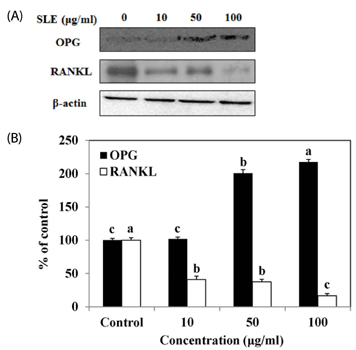
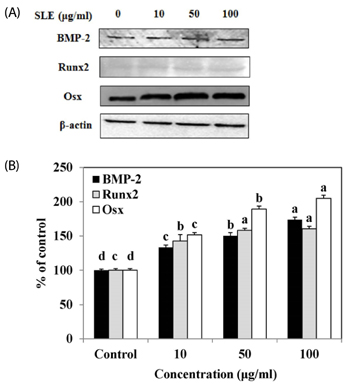
The results indicated that treatment of SLE promoted the proliferation of MC3T3-E1 cells and improved ALP activity. And, SLE treatment significantly promoted mineralized nodule formation compared with control. In addition, cells treated with SLE significantly upregulated protein expression of ALP, type 1 collagen, bone morphogenetic protein 2, runt-related transcription factor 2, osterix, and osteoprotegerin.
CONCLUSIONS
The results demonstrate that SLE promote differentiation inducement and proliferation of osteoblasts and, therefore may help to elucidate the transcriptional mechanism of bone formation and possibly lead to the development of bone-forming drugs.
Keyword
MeSH Terms
Figure
Reference
-
1. Michaëlsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL. Genetic liability to fractures in the elderly. Arch Intern Med. 2005; 165:1825–1830.
Article2. Slemenda CW, Christian JC, Williams CJ, Norton JA, Johnston CC Jr. Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991; 6:561–567.
Article3. Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002; 23:279–302.
Article4. Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, Takahashi K, Nishihira J, Izumi H, Sakamoto W. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology. 2004; 197:93–100.
Article5. Yonezawa T, Hasegawa S, Asai M, Ninomiya T, Sasaki T, Cha BY, Teruya T, Ozawa H, Yagasaki K, Nagai K, Woo JT. Harmine, a β-carboline alkaloid, inhibits osteoclast differentiation and bone resorption in vitro and in vivo. Eur J Pharmacol. 2011; 650:511–518.
Article6. Lane NE, Kelman A. A review of anabolic therapies for osteoporosis. Arthritis Res Ther. 2003; 5:214–222.7. Yoon HJ, Seo CR, Kim M, Kim YJ, Song NJ, Jang WS, Kim BJ, Lee J, Hong JW, Nho CW, Park KW. Dichloromethane extracts of Sophora japonica L. stimulate osteoblast differentiation in mesenchymal stem cells. Nutr Res. 2013; 33:1053–1062.
Article8. Zhang R, Oyajobi BO, Harris SE, Chen D, Tsao C, Deng HW, Zhao M. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013; 52:145–156.
Article9. Demirel Z, Yilmaz-Koz FF, Karabay-Yavasoglu UN, Ozdemir G, Sukatar A. Antimicrobial and antioxidant activity of brown algae from the Aegean sea. J Serb Chem Soc. 2009; 74:619–628.
Article10. Nakamura T, Nagayama K, Kawaguchi S. High tocopherol content in a brown alga Ishige okamurae. Fish Sci. 1994; 60:793–794.11. Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology. 1997; 138:3849–3858.
Article12. Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, Shuey D, Zygowicz ER. A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem. 1983; 29:751–761.
Article13. Whyte MP. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994; 15:439–461.
Article14. Niu YB, Li YH, Kong XH, Zhang R, Sun Y, Li Q, Li C, Liu L, Wang J, Mei QB. The beneficial effect of Radix Dipsaci total saponins on bone metabolism in vitro and in vivo and the possible mechanisms of action. Osteoporos Int. 2012; 23:2649–2660.
Article15. Musiał K, Fornalczyk K, Zwolińska D. [Osteopontin (OPN), PDGF-BB (platelet-derived growth factor) and BMP-7 (bone morphogenetic protein) as markers of atherogenesis in children with chronic kidney disease (CKD) treated conservatively--preliminary results]. Pol Merkur Lekarski. 2008; 24:Suppl 4. 25–27.16. W W. Glial line-derived neurotrophic factor (GDNF): biological activities. Folia Morphol (Warsz). 1999; 58:155–159.17. Li F, Yang Y, Zhu P, Chen W, Qi D, Shi X, Zhang C, Yang Z, Li P. Echinacoside promotes bone regeneration by increasing OPG/RANKL ratio in MC3T3-E1 cells. Fitoterapia. 2012; 83:1443–1450.
Article18. Lee HS, Jung EY, Bae SH, Kwon KH, Kim JM, Suh HJ. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by yeast hydrolysate. Phytother Res. 2011; 25:716–723.
Article19. Christiansen C. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993; 94:646–650.
Article20. Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000; 289:1508–1514.
Article21. Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000; 289:1501–1504.
Article22. Hsieh TP, Sheu SY, Sun JS, Chen MH, Liu MH. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine. 2010; 17:414–423.
Article23. Chandini SK, Ganesan P, Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008; 107:707–713.
Article24. Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun. 2004; 314:197–207.
Article25. Neve A, Corrado A, Cantatore FP. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011; 343:289–302.
Article26. Bellows CG, Aubin JE, Heersche JN. Initiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphate. Bone Miner. 1991; 14:27–40.
Article27. Horiguchi Y, Nakai T, Kume K. Effects of Bordetella bronchiseptica dermonecrotic toxin on the structure and function of osteoblastic clone MC3T3-E1 cells. Infect Immun. 1991; 59:1112–1116.
Article28. Kühn MC, Willenberg HS, Schott M, Papewalis C, Stumpf U, Flohé S, Scherbaum WA, Schinner S. Adipocyte-secreted factors increase osteoblast proliferation and the OPG/RANKL ratio to influence osteoclast formation. Mol Cell Endocrinol. 2012; 349:180–188.
Article29. Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997; 89:309–319.
Article30. Park KH, Kang JW, Lee EM, Kim JS, Rhee YH, Kim M, Jeong SJ, Park YG, Kim SH. Melatonin promotes osteoblastic differentiation through the BMP/ERK/Wnt signaling pathways. J Pineal Res. 2011; 51:187–194.
Article31. Huang W, Rudkin GH, Carlsen B, Ishida K, Ghasri P, Anvar B, Yamaguchi DT, Miller TA. Overexpression of BMP-2 modulates morphology, growth, and gene expression in osteoblastic cells. Exp Cell Res. 2002; 274:226–234.
Article32. Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006; 21:637–646.
Article33. Liu ZP, Li WX, Yu B, Huang J, Sun J, Huo JS, Liu CX. Effects of trans-resveratrol from Polygonum cuspidatum on bone loss using the ovariectomized rat model. J Med Food. 2005; 8:14–19.
Article34. Rassi CM, Lieberherr M, Chaumaz G, Pointillart A, Cournot G. Down-regulation of osteoclast differentiation by daidzein via caspase 3. J Bone Miner Res. 2002; 17:630–638.
Article35. O'Gorman DM, Tierney CM, Brennan O, O'Brien FJ. The marinederived, multi-mineral formula, Aquamin, enhances mineralisation of osteoblast cells in vitro. Phytother Res. 2012; 26:375–380.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lactoferrin Constitutively Enhances Differentiation of Osteoblastic MC3T3-E1 Cells in Vitro
- Effect of Sambucus sieboldiana Extract on the Cell Growth and Extracellular Matrix Formation in Osteoblast Cells
- Glycyrrhiza uralensis (licorice) extracts increase cell proliferation and bone marker enzyme alkaline phosphatase activity in osteoblastic MC3T3-E1 cells
- Effects of 2-deoxy-D-glucose and quercetin on the gene expression of bone sialoprotein and osteocalcin during the differentiation in irradiated MC3T3-E1 osteoblastic cells
- On the Activity of MC3T3-E1 Cell in vitro