Anat Cell Biol.
2024 Mar;57(1):85-96. 10.5115/acb.23.159.
Inhibitory effect of temozolomide on apoptosis induction of cinnamaldehyde in human glioblastoma multiforme T98G cell line
- Affiliations
-
- 1Department of Anatomy, Cellular and Molecular Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 2Department of Pharmaceutical Biotechnology, School of Pharmacy, Guilan University of Medical Sciences, Rasht, Iran
- 3Department of Physiology, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- 4Neuroscience Research Center, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
- KMID: 2554243
- DOI: http://doi.org/10.5115/acb.23.159
Abstract
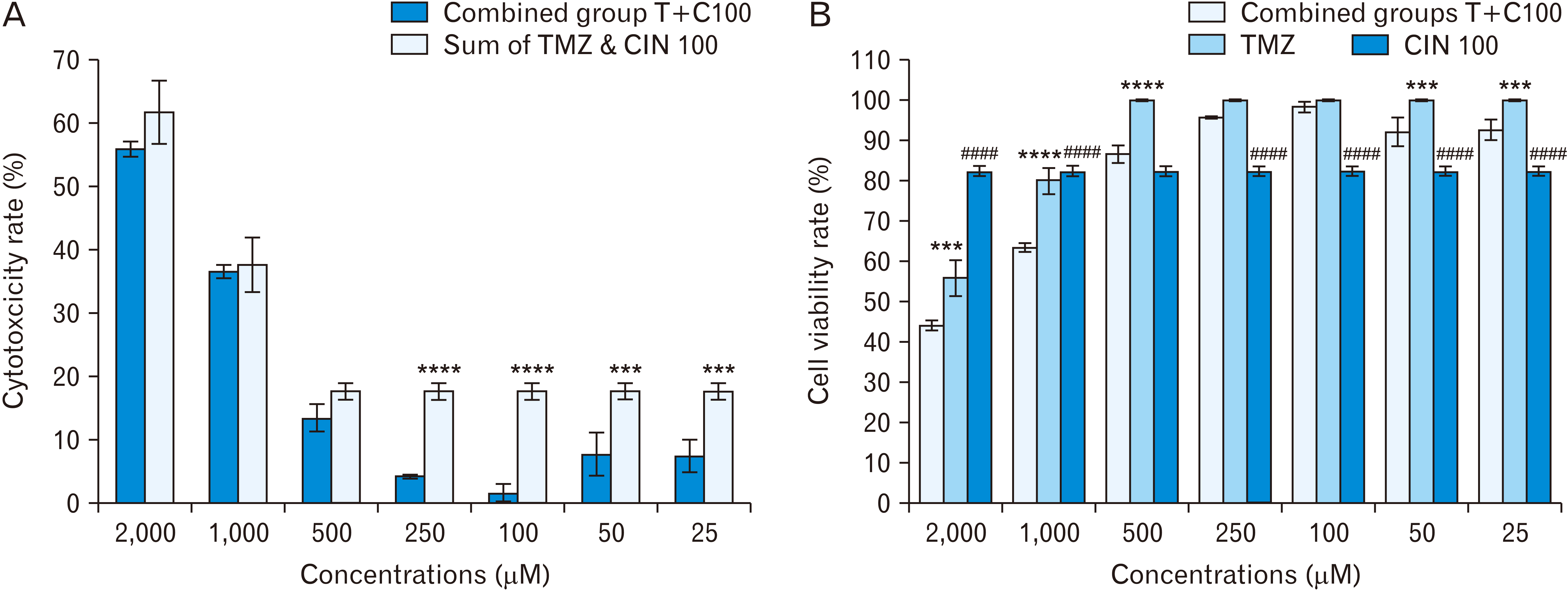
- Glioblastoma is the most common primary malignant brain tumor in adults. Temozolomide (TMZ) is an FDAapproved drug used to treat this type of cancer. Cinnamaldehyde (CIN) is a derivative of cinnamon extract and makes up 99% of it. The aim of this study was to investigate the in vitro combined effect of CIN and TMZ on human glioblastoma multiforme T98G cell line viability. In this study, we used 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl-tertazolium bromide (MTT) method to evaluate the extent of IC50 , acridine orange, Giemsa and Hoechst staining to evaluate the manner of apoptosis and the Western blotting method to examine the expression change of apoptotic proteins. Our results show that TMZ has an inhibitory effect on CIN when both used in combination at concentrations of 300 and 100 μM (P<0.05) and has a cytotoxic effect when used alone at the same concentrations (P<0.05). The western blotting result showed that TMZ at concentrations of 2,000 and 1,000 μM significantly increased Bax expression and decreased Bcl2 expression (P<0.05), indicating that TMZ induced apoptosis through the mitochondrial pathway. However, CIN had no effect on Bax and Bcl2 expressions, thus causing apoptosis from another pathway. Also, the Bax:Bcl2 expression ratio at concentrations combined was lower than that for TMZ 1,000 μM and higher than that for CIN 150 and 100 μM (P<0.05), which confirms the inhibitory effect of TMZ on CIN. From the present study, we conclude that TMZ in combination with CIN has an inhibitory effect on increasing the cytotoxicity rate.
Keyword
Figure
Reference
-
References
1. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. 2018; CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 20(suppl_4):iv1–86. Erratum in: Neuro Oncol 2021;23:508-22. DOI: 10.1093/neuonc/noy131. PMID: 30445539. PMCID: PMC6129949.
Article2. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. 2017; Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 18:3–9.3. Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. 2014; CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 16(Suppl 4):iv1–63. DOI: 10.1093/neuonc/nou223. PMID: 25304271. PMCID: PMC4193675.
Article4. Preusser M, de Ribaupierre S, Wöhrer A, Erridge SC, Hegi M, Weller M, Stupp R. 2011; Current concepts and management of glioblastoma. Ann Neurol. 70:9–21. DOI: 10.1002/ana.22425. PMID: 21786296.
Article5. Stupp R, Brada M, van den Bent MJ, Tonn JC, Pentheroudakis G. 2014; High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 25 Suppl 3:iii93–101. DOI: 10.1093/annonc/mdu050. PMID: 24782454.
Article6. Lee SY. 2016; Temozolomide resistance in glioblastoma multiforme. Genes Dis. 3:198–210. DOI: 10.1016/j.gendis.2016.04.007. PMID: 30258889. PMCID: PMC6150109.
Article7. Alonso MM, Gomez-Manzano C, Bekele BN, Yung WK, Fueyo J. 2007; Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res. 67:11499–504. DOI: 10.1158/0008-5472.CAN-07-5312. PMID: 18089777.
Article8. Ramirez YP, Mladek AC, Phillips RM, Gynther M, Rautio J, Ross AH, Wheelhouse RT, Sakaria JN. 2015; Evaluation of novel imidazotetrazine analogues designed to overcome temozolomide resistance and glioblastoma regrowth. Mol Cancer Ther. 14:111–9. DOI: 10.1158/1535-7163.MCT-14-0113. PMID: 25351918. PMCID: PMC4297195.9. Park I, Mukherjee J, Ito M, Chaumeil MM, Jalbert LE, Gaensler K, Ronen SM, Nelson SJ, Pieper RO. 2014; Changes in pyruvate metabolism detected by magnetic resonance imaging are linked to DNA damage and serve as a sensor of temozolomide response in glioblastoma cells. Cancer Res. 74:7115–24. DOI: 10.1158/0008-5472.CAN-14-0849. PMID: 25320009. PMCID: PMC4253720.
Article10. Smalley S, Chalmers AJ, Morley SJ. 2014; mTOR inhibition and levels of the DNA repair protein MGMT in T98G glioblastoma cells. Mol Cancer. 13:144. DOI: 10.1186/1476-4598-13-144. PMID: 24909675. PMCID: PMC4061125.
Article11. Cen L, Carlson BL, Pokorny JL, Mladek AC, Grogan PT, Schroeder MA, Decker PA, Anderson SK, Giannini C, Wu W, Ballman KV, Kitange GJ, Sarkaria JN. 2013; Efficacy of protracted temozolomide dosing is limited in MGMT unmethylated GBM xenograft models. Neuro Oncol. 15:735–46. DOI: 10.1093/neuonc/not010. PMID: 23479134. PMCID: PMC3661094.
Article12. Melguizo C, Prados J, González B, Ortiz R, Concha A, Alvarez PJ, Madeddu R, Perazzoli G, Oliver JA, López R, Rodríguez-Serrano F, Aránega A. 2012; MGMT promoter methylation status and MGMT and CD133 immunohistochemical expression as prognostic markers in glioblastoma patients treated with temozolomide plus radiotherapy. J Transl Med. 10:250. DOI: 10.1186/1479-5876-10-250. PMID: 23245659. PMCID: PMC3551841.
Article13. Sangal A. 2011; Role of cinnamon as beneficial antidiabetic food adjunct: a review. Adv Appl Sci Res. 2:440–50.14. Vangalapati M, Sree Satya N, Surya Prakash DV, Avanigadda S. 2012; A review on pharmacological activities and clinical effects of cinnamon species. Res J Pharm Biol Chem Sci. 3:653–63.15. Koh WS, Yoon SY, Kwon BM, Jeong TC, Nam KS, Han MY. 1998; Cinnamaldehyde inhibits lymphocyte proliferation and modulates T-cell differentiation. Int J Immunopharmacol. 20:643–60. DOI: 10.1016/S0192-0561(98)00064-2. PMID: 9848396.
Article16. Kwon BM, Lee SH, Choi SU, Park SH, Lee CO, Cho YK, Sung ND, Bok SH. 1998; Synthesis and in vitro cytotoxicity of cinnamaldehydes to human solid tumor cells. Arch Pharm Res. 21:147–52. DOI: 10.1007/BF02974019. PMID: 9875422.17. Shaughnessy DT, Setzer RW, DeMarini DM. The antimutagenic effect of vanillin and cinnamaldehyde on spontaneous mutation in Salmonella TA104 is due to a reduction in mutations at GC but not AT sites. Mutat Res. 2001; 480-481:55–69. DOI: 10.1016/S0027-5107(01)00169-5. PMID: 11506799.
Article18. Ka H, Park HJ, Jung HJ, Choi JW, Cho KS, Ha J, Lee KT. 2003; Cinnamaldehyde induces apoptosis by ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Cancer Lett. 196:143–52. DOI: 10.1016/S0304-3835(03)00238-6. PMID: 12860272.
Article19. Ng LT, Wu SJ. 2011; Antiproliferative activity of cinnamomum cassia constituents and effects of pifithrin-alpha on their apoptotic signaling pathways in Hep G2 cells. Evid Based Complement Alternat Med. 2011:492148. DOI: 10.1093/ecam/nep220. PMID: 20038571. PMCID: PMC3135661.20. Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. 2003; Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 63:2103–8.21. Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. 2004; Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 11:448–57. DOI: 10.1038/sj.cdd.4401359. PMID: 14713959.
Article22. Jakubowicz-Gil J, Langner E, Bądziul D, Wertel I, Rzeski W. 2013; Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 34:2367–78. DOI: 10.1007/s13277-013-0785-0. PMID: 23580181. PMCID: PMC3713258.
Article23. Pasi F, Persico MG, Buroni FE, Aprile C, Hodolic M, Corbella F, Nano R, Facoetti A, Lodola L. 2017; Uptake of 18F-FET and 18F-FCH in human glioblastoma T98G cell line after irradiation with photons or carbon ions. Contrast Media Mol Imaging. 2017:6491674. DOI: 10.1155/2017/6491674. PMID: 29097931. PMCID: PMC5612615.24. Niknezhad F, Sayad-Fathi S, Karimzadeh A, Ghorbani-Anarkooli M, Yousefbeyk F, Nasiri E. 2019; Improvement in histology, enzymatic activity, and redox state of the liver following administration of Cinnamomum zeylanicum bark oil in rats with established hepatotoxicity. Anat Cell Biol. 52:302–11. DOI: 10.5115/acb.18.180. PMID: 31598360. PMCID: PMC6773892.
Article25. Figul M, Söling A, Dong HJ, Chou TC, Rainov NG. 2003; Combined effects of temozolomide and the ribonucleotide reductase inhibitors didox and trimidox in malignant brain tumor cells. Cancer Chemother Pharmacol. 52:41–6. DOI: 10.1007/s00280-003-0611-2. PMID: 12690517.
Article26. Chen JC, Hsieh PS, Chen SM, Hwang JH. 2020; Effects of cinnamaldehyde on the viability and expression of chemokine receptor genes in temozolomide-treated glioma cells. In Vivo. 34:595–9. DOI: 10.21873/invivo.11812. PMID: 32111758. PMCID: PMC7157882.
Article27. Wu SJ, Ng LT, Lin CC. 2005; Cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells through activation of the proapoptotic Bcl-2 family proteins and MAPK pathway. Life Sci. 77:938–51. DOI: 10.1016/j.lfs.2005.02.005. PMID: 15964311.
Article28. Wu SJ, Ng LT. 2007; MAPK inhibitors and pifithrin-alpha block cinnamaldehyde-induced apoptosis in human PLC/PRF/5 cells. Food Chem Toxicol. 45:2446–53. DOI: 10.1016/j.fct.2007.05.032. PMID: 17673346.
Article29. Wu SJ, Ng LT, Lin CC. 2004; Effects of vitamin E on the cinnamaldehyde-induced apoptotic mechanism in human PLC/PRF/5 cells. Clin Exp Pharmacol Physiol. 31:770–6. DOI: 10.1111/j.1440-1681.2004.04091.x. PMID: 15566391.
Article30. Zhang JH, Liu LQ, He YL, Kong WJ, Huang SA. 2010; Cytotoxic effect of trans-cinnamaldehyde on human leukemia K562 cells. Acta Pharmacol Sin. 31:861–6. DOI: 10.1038/aps.2010.76. PMID: 20581850. PMCID: PMC4007726.
Article31. Grootjans S, Hassannia B, Delrue I, Goossens V, Wiernicki B, Dondelinger Y, Bertrand MJ, Krysko DV, Vuylsteke M, Vandenabeele P, Vanden Berghe T. 2016; A real-time fluorometric method for the simultaneous detection of cell death type and rate. Nat Protoc. 11:1444–54. DOI: 10.1038/nprot.2016.085. PMID: 27414760.
Article32. Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. 2008; Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 135:1311–23. DOI: 10.1016/j.cell.2008.10.044. PMID: 19109899. PMCID: PMC2621059.
Article33. Richards R, Schwartz HR, Honeywell ME, Stewart MS, Cruz-Gordillo P, Joyce AJ, Landry BD, Lee MJ. 2020; Drug antagonism and single-agent dominance result from differences in death kinetics. Nat Chem Biol. 16:791–800. DOI: 10.1038/s41589-020-0510-4. PMID: 32251407. PMCID: PMC7311243.
Article34. Chio CC, Tai YT, Mohanraj M, Liu SH, Yang ST, Chen RM. 2018; Honokiol enhances temozolomide-induced apoptotic insults to malignant glioma cells via an intrinsic mitochondrion-dependent pathway. Phytomedicine. 49:41–51. DOI: 10.1016/j.phymed.2018.06.012. PMID: 30217261.
Article35. Sur P, Sribnick EA, Patel SJ, Ray SK, Banik NL. 2005; Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia. 50:160–7. DOI: 10.1002/glia.20168. PMID: 15685605.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cisplatin-Induced Apoptosis by Cisplatin in Human Glioblastoma Cell Line
- Midkine Expression in Cell Lines and tumor Tissues of Glioblastoma Multiforme
- A Case of Meningioma Compatible with Metastatic Glioblastoma Multiforme
- A Case of Multicentric Glioblastoma Multiforme
- Disulfiram, a Re-positioned Aldehyde Dehydrogenase Inhibitor, Enhances Radiosensitivity of Human Glioblastoma Cells In Vitro