Ann Lab Med.
2024 Mar;44(2):155-163. 10.3343/alm.2023.0212.
Head-to-Head Comparison of Nine Assays for the Detection of Anti-Echinococcus Antibodies: A Retrospective Evaluation
- Affiliations
-
- 1Max von Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie, Medizinische Fakultät, LMU München, Munich, Germany
- 2Division of Infectious Diseases and Tropical Medicine, University Hospital Ludwig-Maximilian University Munich, Munich, Germany
- 3German Centre for Infection Research (DZIF), Munich, Germany
- 4Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Immunology, Infection and Pandemic Research, Munich, Germany
- 5Diagnostic and Research Institute of Hygiene, Microbiology and Environmental Medicine, Medical University of Graz, Graz, Austria
- KMID: 2553384
- DOI: http://doi.org/10.3343/alm.2023.0212
Abstract
- Background
Echinococcosis is a neglected tropical disease that is severely underdiagnosed in resource-limited settings. In developed countries, diagnosing echinococcosis is challenging, and reliable serological assays are urgently needed. In the Central European Alps, EM is more common than EG; however, data on the diagnostic performance of assays for EM cases are scarce. We evaluated the suitability of nine antibody assays for routine diagnostics.
Methods
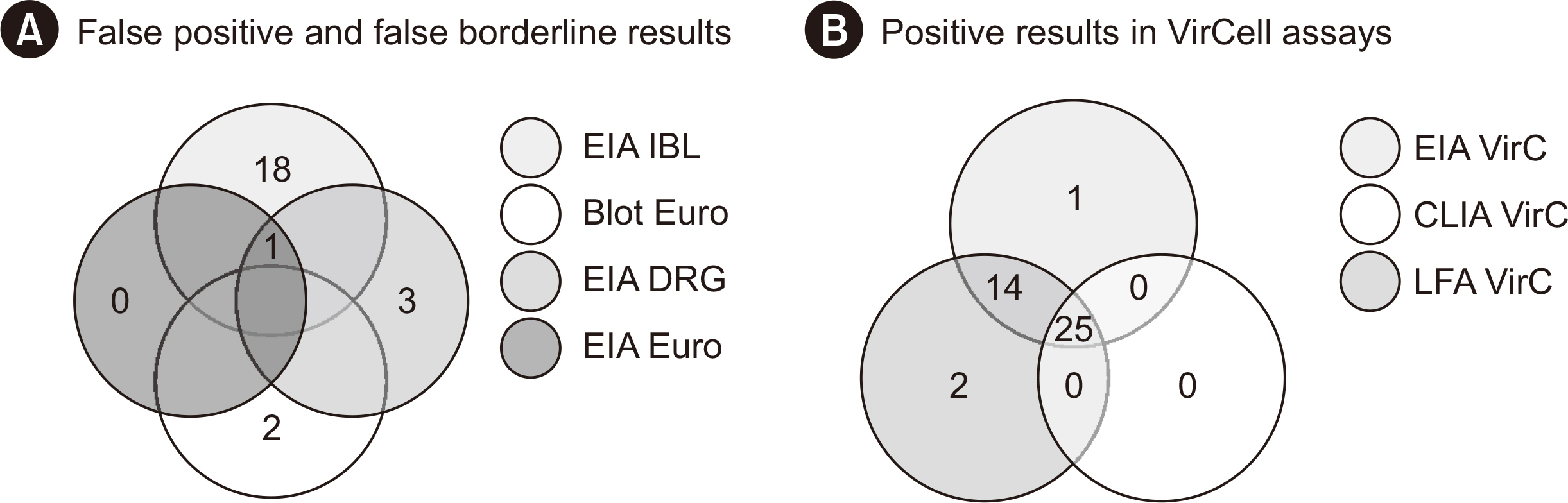
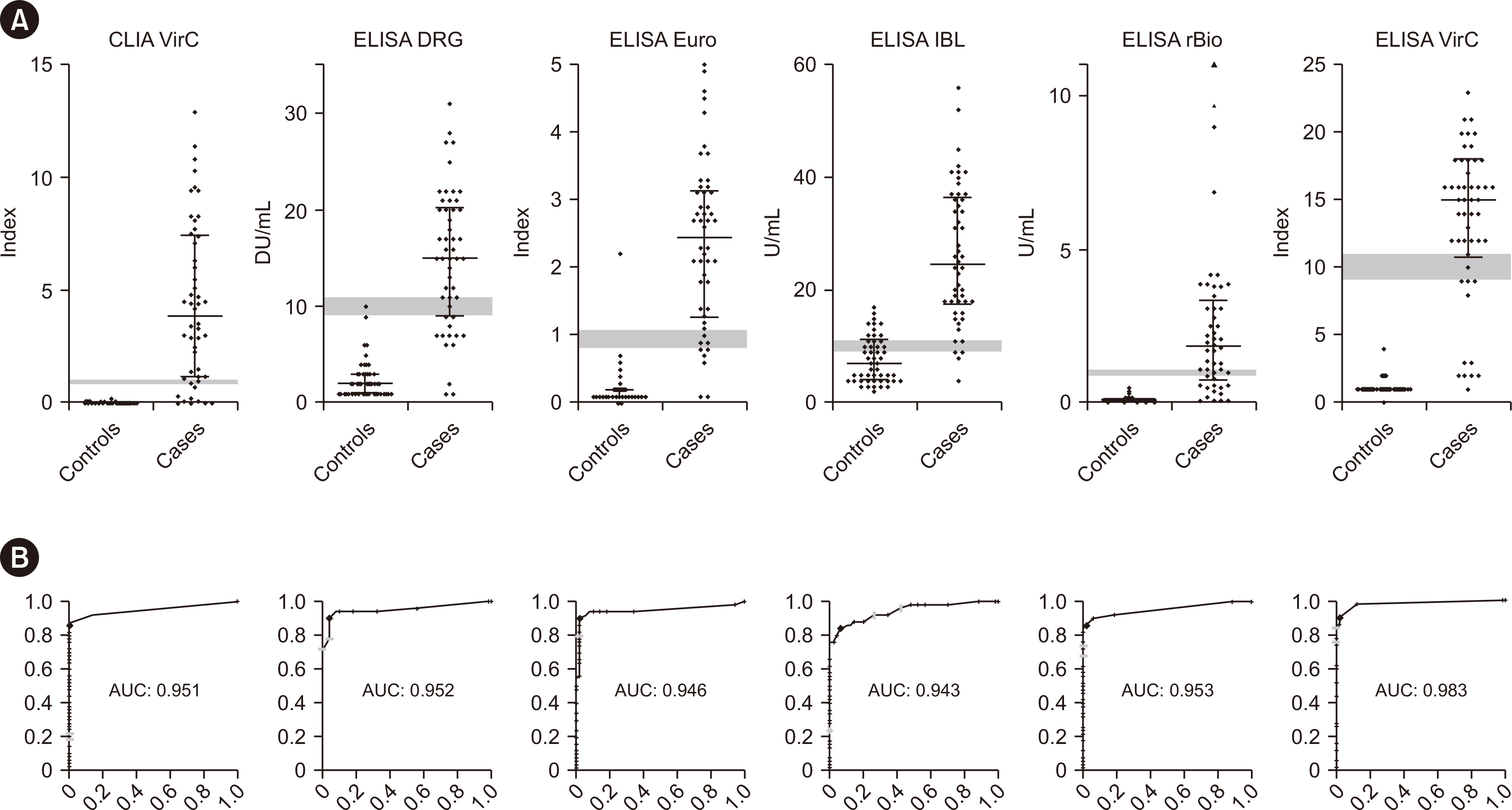
Nine commercially available serological assays for detecting anti-Echinococcus antibodies were compared head-to-head using samples collected from 50 patients with echinococcosis and 50 age- and sex-matched control subjects. The assays are Anti-Echinococcus ELISA (IgG) (Euroimmun), Echinococcus IgG ELISA (DRG), Echinococcus IgG ELISA (IBL International), Echinococcus Western Blot IgG (LDBIO Diagnostics), EUROLINE WB (Euroimmun), Hydatidosis ELISA IgG (VirCell), Hydatidosis VIRCLIA IgG Monotest (VirCell), Ridascreen Echinococcus IgG (R-Biopharm), and Virapid Hydatidosis (VirCell). The cases were ranked according to the WHO-Informal Working Group on Echinococcosis (WHO-IWGE) criteria as confirmed, probable, or possible.
Results
The performance of the assays varied greatly, with overall sensitivities ranging between 50% and 88% and specificities between 62% and 100%. We observed a trend toward better performance with cases classified as “confirmed” using the WHO-IWGE criteria. Combined analysis with sequential screening and confirmatory testing resulted in a maximum sensitivity of 84% and specificity of 100%. Differentiation between EG and EM infections is clinically relevant but was found to be unreliable.
Conclusions
Echinococcus serological assays are highly variable in terms of sensitivity and specificity. Knowledge of the pre-test probability in the patient cohort is required to choose a suitable assay. A combined approach with screening and confirmatory assays may be the best diagnostic strategy in many situations.
Keyword
Figure
Reference
-
1. Woolsey ID, Miller AL. 2021; Echinococcus granulosus sensu lato and Echinococcus multilocularis: a review. Res Vet Sci. 135:517–22. DOI: 10.1016/j.rvsc.2020.11.010. PMID: 33246571.2. Baumann S, Shi R, Liu W, Bao H, Schmidberger J, Kratzer W, et al. 2019; Worldwide literature on epidemiology of human alveolar echinococcosis: a systematic review of research published in the twenty-first century. Infection. 47:703–27. DOI: 10.1007/s15010-019-01325-2. PMID: 31147846. PMCID: PMC8505309.
Article3. Casulli A, Abela-Ridder B, Petrone D, Fabiani M, Bobić B, Carmena D, et al. 2023; Unveiling the incidences and trends of the neglected zoonosis cystic echinococcosis in Europe: a systematic review from the MEmE project. Lancet Infect Dis. 23:e95–107. DOI: 10.1016/S1473-3099(22)00638-7. PMID: 36427513.
Article4. Casulli A. 2020; Recognising the substantial burden of neglected pandemics cystic and alveolar echinococcosis. Lancet Glob Health. 8:e470–1. DOI: 10.1016/S2214-109X(20)30066-8. PMID: 32199112.
Article5. Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, et al. 2019; Echinococcosis: advances in the 21st century. Clin Microbiol Rev. 32:e00075–18. DOI: 10.1128/CMR.00075-18. PMID: 30760475. PMCID: PMC6431127.
Article6. Karshima SN, Ahmed MI, Adamu NB, Magaji AA, Zakariah M, Mohammed K. 2022; Africa-wide meta-analysis on the prevalence and distribution of human cystic echinococcosis and canine Echinococcus granulosus infections. Parasit Vectors. 15:357. DOI: 10.1186/s13071-022-05474-6. PMID: 36199100. PMCID: PMC9535855.7. Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE. 2010; Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 114:1–16. DOI: 10.1016/j.actatropica.2009.11.001. PMID: 19931502.
Article8. Eckert J, Deplazes P. 2004; Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev. 17:107–35. DOI: 10.1128/CMR.17.1.107-135.2004. PMID: 14726458. PMCID: PMC321468.
Article9. Budke CM, Deplazes P, Torgerson PR. 2006; Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 12:296–303. DOI: 10.3201/eid1202.050499. PMID: 16494758. PMCID: PMC3373106.
Article10. Torgerson PR, Keller K, Magnotta M, Ragland N. 2010; The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 4:e722. DOI: 10.1371/journal.pntd.0000722. PMID: 20582310. PMCID: PMC2889826.
Article11. Vuitton DA. 2004; Echinococcosis and allergy. Clin Rev Allergy Immunol. 26:93–104. DOI: 10.1007/s12016-004-0004-2. PMID: 15146106.
Article12. Calame P, Weck M, Busse-Cote A, Brumpt E, Richou C, Turco C, et al. 2022; Role of the radiologist in the diagnosis and management of the two forms of hepatic echinococcosis. Insights Imaging. 13:68. DOI: 10.1186/s13244-022-01190-y. PMID: 35394226. PMCID: PMC8994011.
Article13. Stojković M, Weber TF, Junghanss T. 2018; Clinical management of cystic echinococcosis: state of the art and perspectives. Curr Opin Infect Dis. 31:383–92. DOI: 10.1097/QCO.0000000000000485. PMID: 30124496.
Article14. Tamarozzi F, Silva R, Fittipaldo VA, Buonfrate D, Gottstein B, Siles-Lucas M. 2021; Serology for the diagnosis of human hepatic cystic echinococcosis and its relation with cyst staging: a systematic review of the literature with meta-analysis. PLoS Negl Trop Dis. 15:e0009370. DOI: 10.1371/journal.pntd.0009370. PMID: 33909640. PMCID: PMC8081258.
Article15. Tamer GS, Dündar D, Uzuner H, Baydemir C. 2015; Evaluation of immunochromatographic test for the detection of antibodies against Echinococcosis granulosus. Med Sci Monit. 21:1219–22. DOI: 10.12659/MSM.893155. PMID: 25921809. PMCID: PMC4427020.16. Baraquin A, Zait H, Grenouillet FE, Moreau E, Hamrioui B, Grenouillet F. 2017; Large-scale evaluation of a rapid diagnostic test for human cystic echinococcosis. Diagn Microbiol Infect Dis. 89:20–5. DOI: 10.1016/j.diagmicrobio.2017.06.002. PMID: 28647066.
Article17. Peruzzu A, Mastrandrea S, Fancellu A, Bonelli P, Muehlethaler K, Masala G, et al. 2022; Comparison and evaluation of analytic and diagnostic performances of four commercial kits for the detection of antibodies against Echinococcus granulosus and multilocularis in human sera. Comp Immunol Microbiol Infect Dis. 86:101816. DOI: 10.1016/j.cimid.2022.101816. PMID: 35472655.18. Vola A, Manciulli T, De Silvestri A, Lissandrin R, Mariconti M, Siles-Lucas M, et al. 2019; Diagnostic performances of commercial ELISA, indirect hemagglutination, and western blot in differentiation of hepatic echinococcal and non-echinococcal lesions: a retrospective analysis of data from a single referral centre. Am J Trop Med Hyg. 101:1345–9. DOI: 10.4269/ajtmh.19-0556. PMID: 31674293. PMCID: PMC6896875.
Article19. Mahajan S, Thapar S, Khillan V, Gupta P, Rastogi A, Gupta E. 2020; Comparative evaluation of echinococcus serology with cytology for the diagnosis of hepatic hydatid disease. J Lab Physicians. 12:98–102. DOI: 10.1055/s-0040-1716460. PMID: 32905299. PMCID: PMC7467829.
Article20. Tamarozzi F, Longoni SS, Vola A, Degani M, Tais S, Rizzi E, et al. 2021; Evaluation of nine commercial serological tests for the diagnosis of human hepatic cyst echinococcosis and the differential diagnosis with other focal liver lesions: a diagnostic accuracy study. Diagnostics (Basel). 11:167. DOI: 10.3390/diagnostics11020167. PMID: 33503986. PMCID: PMC7911993.
Article21. Deininger S, Wellinghausen N. 2019; Evaluation of a new combined western and line blot assay (EUROLINE-WB) for diagnosis and species identification of Echinococcus infection in humans. GMS Infect Dis. 7:Doc01.22. Hernández-González A, Sánchez-Ovejero C, Manzano-Román R, González Sánchez M, Delgado JM, Pardo-García T, et al. 2018; Evaluation of the recombinant antigens B2t and 2B2t, compared with hydatid fluid, in IgG-ELISA and immunostrips for the diagnosis and follow up of CE patients. PLoS Negl Trop Dis. 12:e0006741. DOI: 10.1371/journal.pntd.0006741. PMID: 30188936. PMCID: PMC6143278.
Article23. Lissandrin R, Tamarozzi F, Piccoli L, Tinelli C, De Silvestri A, Mariconti M, et al. 2016; Factors influencing the serological response in hepatic Echinococcus granulosus infection. Am J Trop Med Hyg. 94:166–71. DOI: 10.4269/ajtmh.15-0219. PMID: 26503271. PMCID: PMC4710424.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation Between C3d Assay and Single Antigen Bead Assay for Detection of Human Leukocyte Antigen Class II Antibodies
- Comparison of the Diagnostic Performance of Elecsys Anti-HCV II and Elecsys and Vitros Anti-HCV Assays
- A Case of Recurred Hydatid Cyst in Pelvic Cavity
- Performance Evaluation of Anti-rubella IgM and IgG Antibodies by Roche Modulddar Analytics E170
- Methods for Detection of Human Papillomavirus in Head and Neck Cancer