Lab Med Online.
2020 Oct;10(4):295-300. 10.47429/lmo.2020.10.4.295.
Correlation Between C3d Assay and Single Antigen Bead Assay for Detection of Human Leukocyte Antigen Class II Antibodies
- Affiliations
-
- 1Department of Laboratory Medicine, Keimyung University School of Medicine, Daegu, Korea
- KMID: 2512268
- DOI: http://doi.org/10.47429/lmo.2020.10.4.295
Abstract
- Background
Detection of anti-human leukocyte antigen (HLA) antibodies is important during the selection of an appropriate donor prior to organ transplantation and also for monitoring the patients after transplantation. In this study, we compared antibodies detected via C3d assays, which monitors C3d complement-binding activities of HLA antibodies with those detected via single antigen bead (SAB) assays.
Methods
A total of 66 serum samples were tested in parallel by SAB assays (Immucor Transplant Diagnostics, USA) and C3d assays (Immucor) for the detection of HLA class II antibodies. The relationship between these two methods was analyzed based on the types, numbers, median fluorescent intensity (MFI) values, and positivity of the antibodies using MATCH IT! Antibody (Immucor) program.
Results
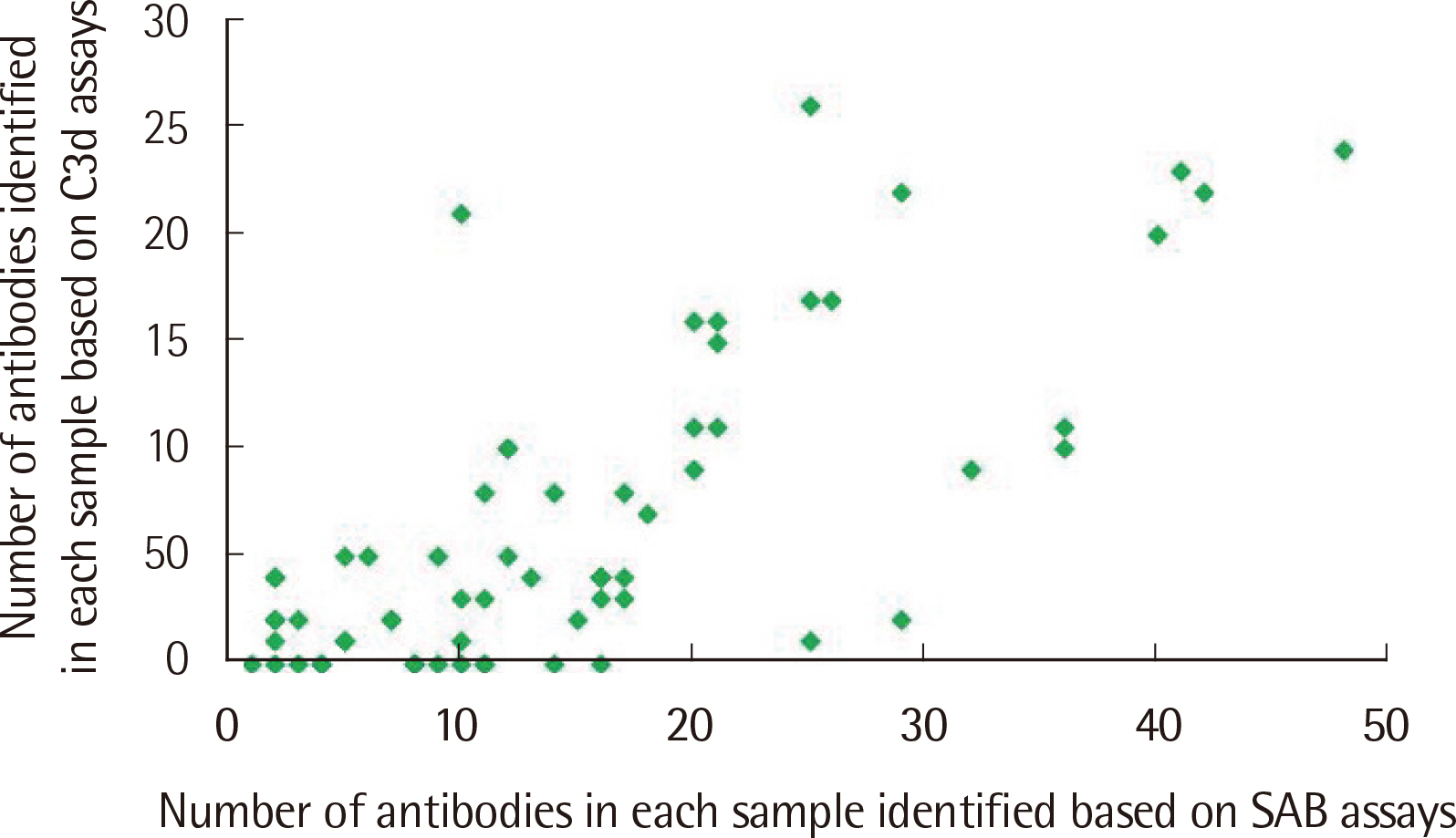
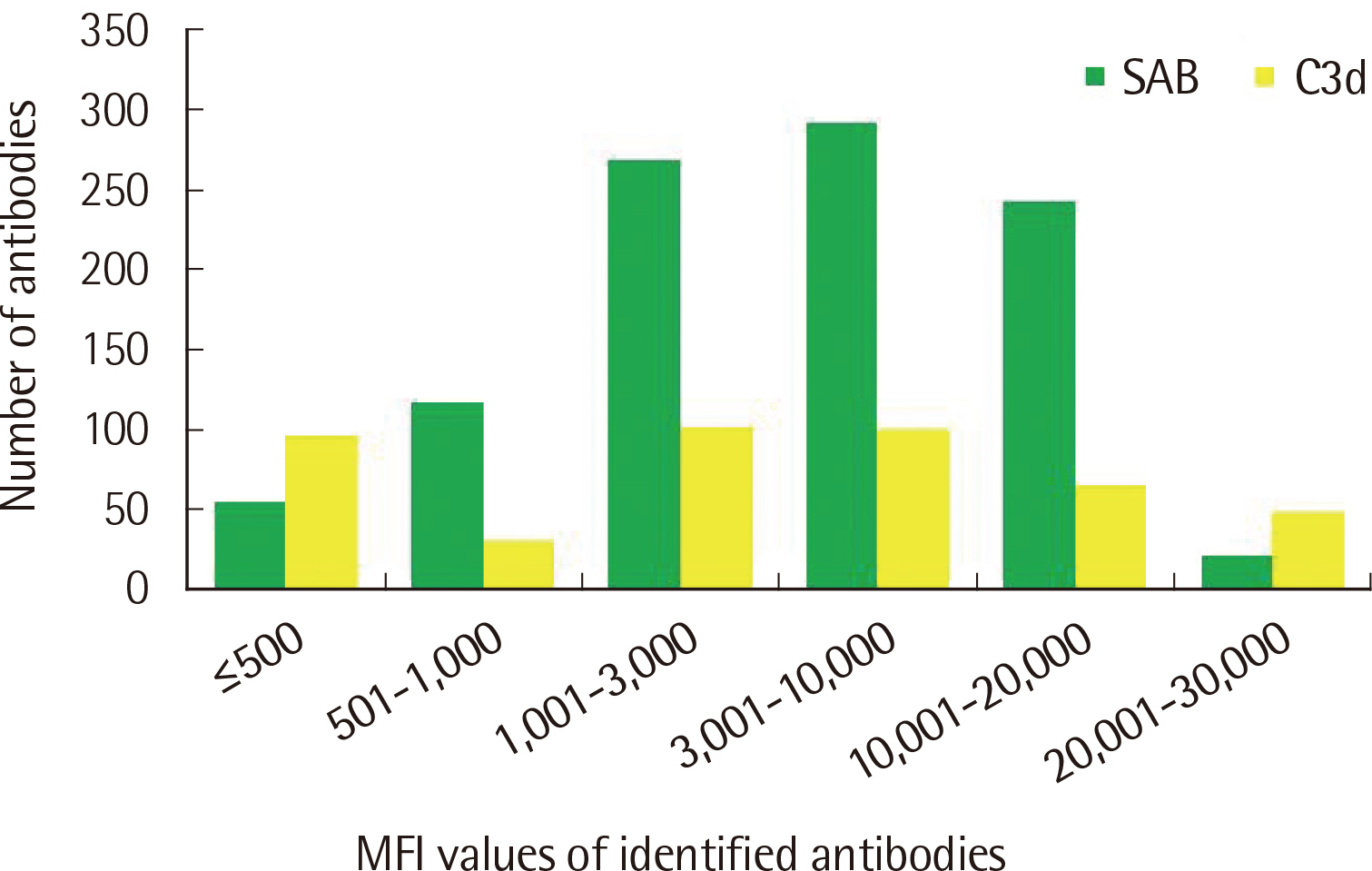
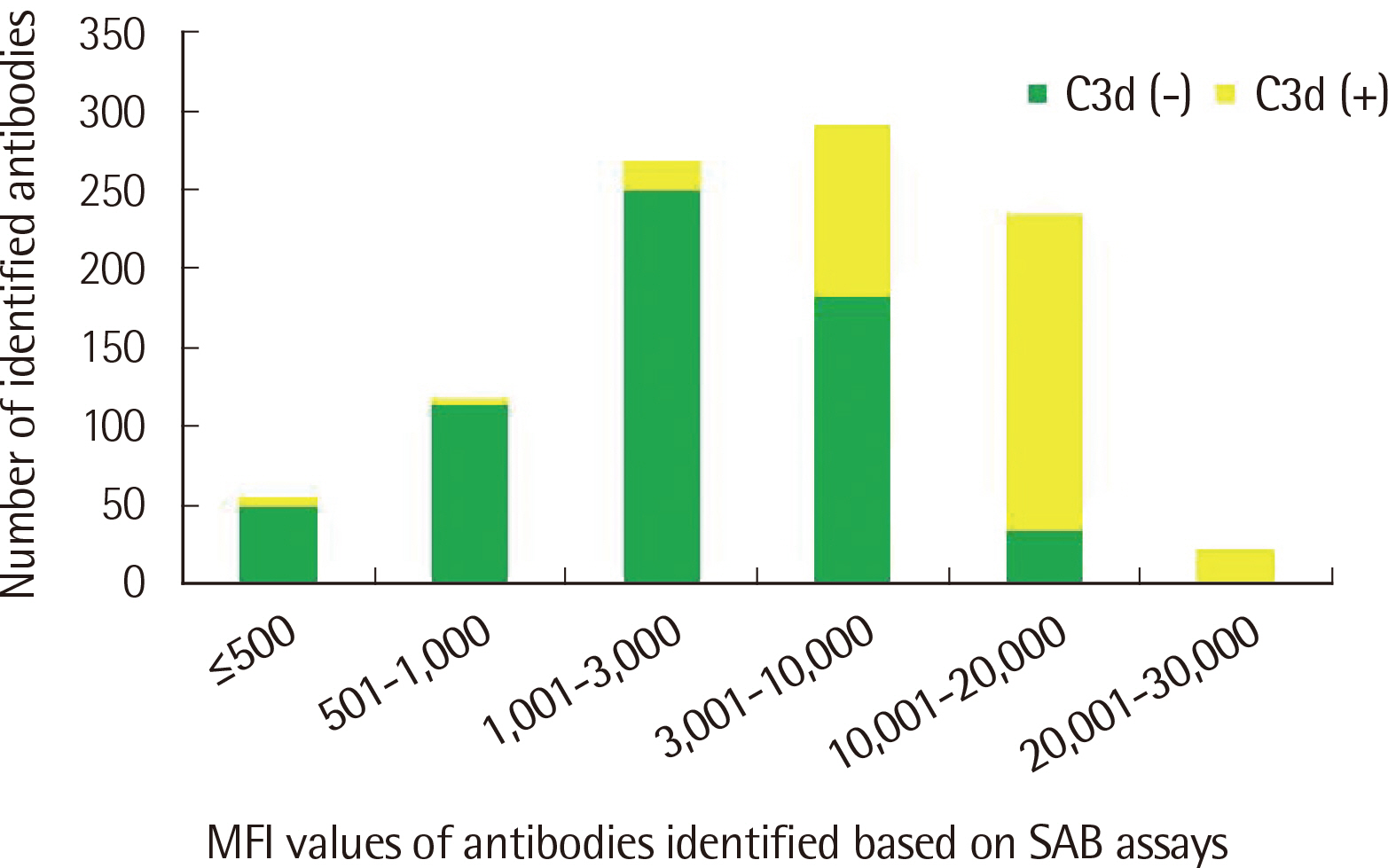
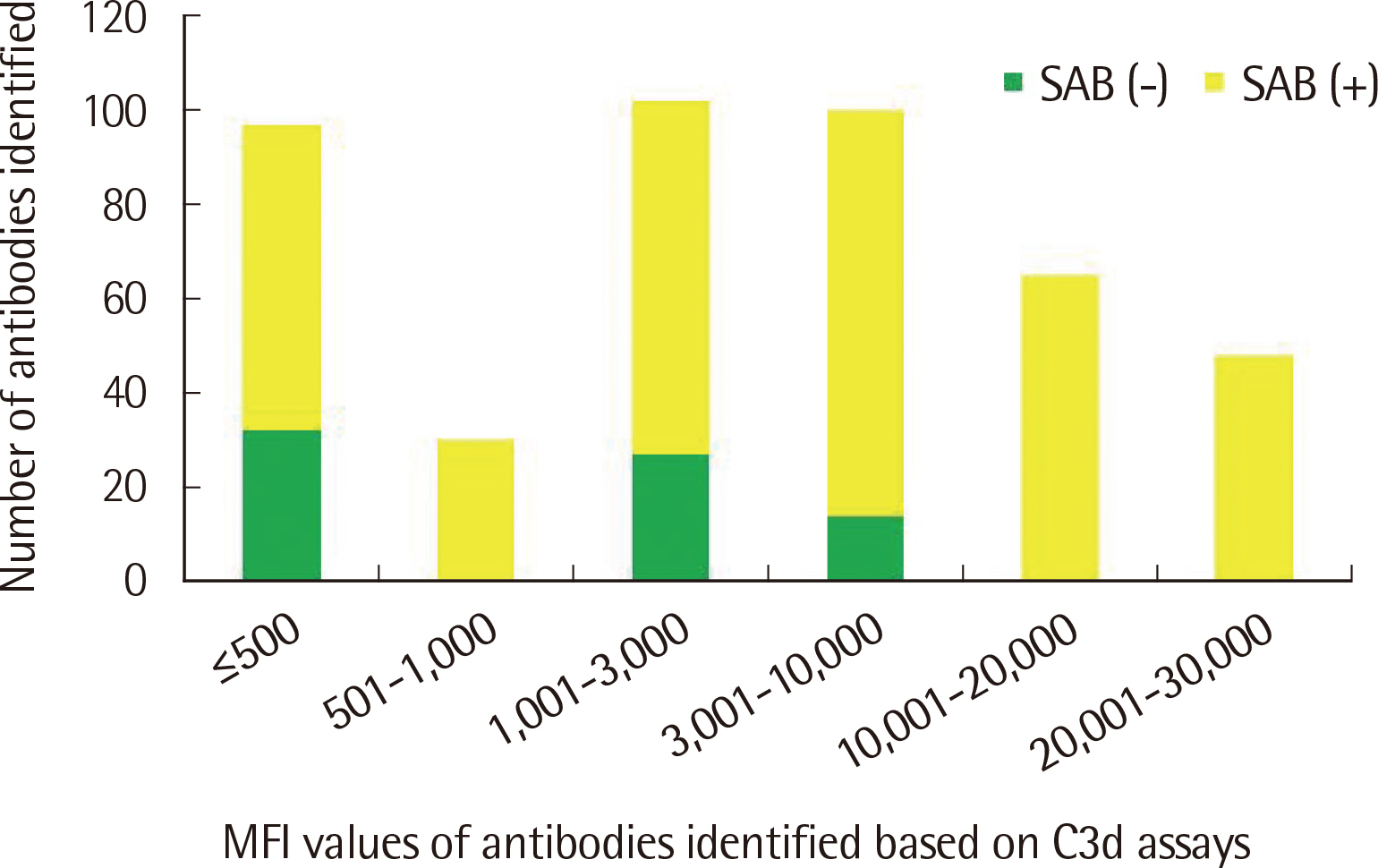
The number of antibodies obtained based on SAB and C3d assays was the highest with 24 samples (36.4%) in the 11–20 range and 23 (34.8%) in the 2–5 range detected via each assay. Among the SAB-positive antibodies, only 28 (6.4%) of the 440 antibodies with MFI ≤3,000 were C3d-positive, and 341 (61.3%) of the 556 antibodies with MFI ≥3,001 were C3d-positive. Whereas, among the 442 C3d-positive antibodies, SAB assays were positive except for 32 (7.2%) and 41 (9.3%) antibodies in the sections of MFI ≤500 and 1,001 ≤MFI ≤10,000, respectively. C3d-positive samples had higher maximum MFI values based on SAB assays, compared with C3d-negative samples.
Conclusions
MFI values of HLA class II antibodies detected through SAB assays in C3d-positive samples were higher than those in C3d-negative samples.
Figure
Reference
-
1. Tinckam KJ, Chandraker A. 2006; Mechanisms and role of HLA and non-HLA alloantibodies. Clin J Am Soc Nephrol. 1:404–14. DOI: 10.2215/CJN.00270106. PMID: 17699239.
Article2. Valenzuela NM, Reed EF. 2013; Antibodies in transplantation: The effects of HLA and non-HLA antibody binding and mechanisms of injury. Methods Mol Biol. 1034:41–70. DOI: 10.1007/978-1-62703-493-7_2. PMID: 23775730. PMCID: PMC3879955.
Article3. Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, et al. 2009; Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 9:2561–70. DOI: 10.1111/j.1600-6143.2009.02813.x. PMID: 19775320.
Article4. Schönemann C, Groth J, Leverenz S, May G. 1998; HLA class I and class II antibodies: monitoring before and after kidney transplantation and their clinical relevance. Transplantation. 65:1519–23. DOI: 10.1097/00007890-199806150-00024. PMID: 9645818.5. Jung S, Oh EJ, Yang CW, Ahn WS, Kim Y, Park YJ, et al. 2009; Comparative evaluation of ELISA and Luminex panel reactive antibody assays for HLA alloantibody screening. Korean J Lab Med. 29:473–80. DOI: 10.3343/kjlm.2009.29.5.473. PMID: 19893358.
Article6. Joo DJ, Huh KH, Kim YS, Yoon SJ, Kim HJ, Sohn SS, et al. 2011; Predictive value of donor specific antibody measured by Luminex single antigen assay for antibody mediated rejection after kidney transplantation. J Korean Soc Transplant. 25:169–75. DOI: 10.4285/jkstn.2011.25.3.169.
Article7. Aubert V, Venetz JP, Pantaleo G, Pascual M. 2009; Low levels of human leukocyte antigen donor-specific antibodies detected by solid phase assay before transplantation are frequently clinically irrelevant. Hum Immunol. 70:580–3. DOI: 10.1016/j.humimm.2009.04.011. PMID: 19375474.
Article8. Vlad G, Ho EK, Vasilescu ER, Colovai AI, Stokes MB, Markowitz GS, et al. 2009; Relevance of different antibody detection methods for the prediction of antibody-mediated rejection and deceased-donor kidney allograft survival. Hum Immunol. 70:589–94. DOI: 10.1016/j.humimm.2009.04.018. PMID: 19375470.
Article9. Zeevi A, Lunz J, Feingold B, Shullo M, Bermudez C, Teuteberg J, et al. 2013; Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 32:98–105. DOI: 10.1016/j.healun.2012.09.021. PMID: 23142561. PMCID: PMC3628631.
Article10. Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. 2013; Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 369:1215–26. DOI: 10.1056/NEJMoa1302506. PMID: 24066742.
Article11. Llorente S, Boix F, Eguia J, López M, Bosch A, Martinez H, et al. 2012; C1q-fixing human leukocyte antigen assay in immunized renal patients: correlation between Luminex SAB-C1q and SAB-IgG. Transplant Proc. 44:2535–7. DOI: 10.1016/j.transproceed.2012.09.084. PMID: 23146446.
Article12. Kamburova EG, Wisse BW, Joosten I, Allebes WA, van der Meer A, Hilbrands LB, et al. 2018; Pretransplant C3d-fixing donor-specific anti-HLA antibodies are not associated with increased risk for kidney graft failure. J Am Soc Nephrol. 29:2279–85. DOI: 10.1681/ASN.2018020205. PMID: 30049681. PMCID: PMC6115667.
Article13. Moreno Gonzales MA, Mitema DG, Smith BH, Schinstock CA, Stegall MD, Wakefield LL, et al. 2017; Comparison between total IgG, C1q, and C3d single antigen bead assays in detecting class I complement-binding anti-HLA antibodies. Transplant Proc. 49:2031–5. DOI: 10.1016/j.transproceed.2017.09.040. PMID: 29149956.
Article14. Lee H, Han E, Choi AR, Ban TH, Chung BH, Yang CW, et al. 2018; Clinical impact of complement (C1q, C3d) binding de novo donor-specific HLA antibody in kidney transplant recipients. PLoS One. 13:e0207434. DOI: 10.1371/journal.pone.0207434. PMID: 30427941. PMCID: PMC6235372.
Article15. Comoli P, Cioni M, Tagliamacco A, Quartuccio G, Innocente A, Fontana I, et al. 2016; Acquisition of C3d-binding activity by de novo donor-specific HLA antibodies correlates with graft loss in nonsensitized pediatric kidney recipients. Am J Transplant. 16:2106–16. DOI: 10.1111/ajt.13700. PMID: 26725780.
Article16. Sicard A, Ducreux S, Rabeyrin M, Couzi L, McGregor B, Badet L, et al. 2015; Detection of C3d-binding donor-specific anti-HLA antibodies at diagnosis of humoral rejection predicts renal graft loss. J Am Soc Nephrol. 26:457–67. DOI: 10.1681/ASN.2013101144. PMID: 25125383. PMCID: PMC4310653.
Article17. Laboratory standardization of reporting HLA typing. http://www.ksdi-lm.org/rang_board/list.html?.num=50&code=lib. Updated on Feb 2017.18. Malheiro J, Santos S, Tafulo S, Dias L, Martins S, Fonseca I, et al. 2018; Detection of complement-binding donor-specific antibodies, not IgG-antibody strength nor C4d status, at antibody-mediated rejection diagnosis is an independent predictor of kidney graft failure. Transplantation. 102:1943–54. DOI: 10.1097/TP.0000000000002265. PMID: 29757900.
Article19. Ko SY, Lee W, Jung CW, Cho Y. 2018; C3d assay in correlation with single antigen bead assay for human leukocyte antigen antibodies. Transplant Proc. 50:2354–8. DOI: 10.1016/j.transproceed.2018.03.056. PMID: 30316357.
Article20. Claisse G, Absi L, Cognasse F, Alamartine E, Mariat C, Maillard N. 2017; Relationship between mean fluorescence intensity and C1q/C3d-fixing capacities of anti-HLA antibodies. Hum Immunol. 78:336–41. DOI: 10.1016/j.humimm.2017.02.003. PMID: 28189573.
Article21. Morales-Buenrostro LE, Terasaki PI, Marino-Vázquez LA, Lee JH, El-Awar N, Alberú J. 2008; "Natural" human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 86:1111–5. DOI: 10.1097/TP.0b013e318186d87b. PMID: 18946350.
Article22. El-Awar N, Lee JH, Tarsitani C, Terasaki PI. 2007; HLA Class I epitopes: recognition of binding sites by mAbs or eluted alloantibody confirmed with single recombinant antigens. Hum Immunol. 68:170–80. DOI: 10.1016/j.humimm.2006.11.006. PMID: 17349872.
Article23. Pelletier RP, Balazs I, Adams P, Rajab A, DiPaola NR, Henry ML. 2018; Clinical utility of C3d binding donor-specific anti-human leukocyte antigen antibody detection by single antigen beads after kidney transplantation-a retrospective study. Transpl Int. 31:424–35. DOI: 10.1111/tri.13106. PMID: 29265514.
Article24. Schwaiger E, Wahrmann M, Bond G, Eskandary F, Böhmig GA. 2014; Complement component C3 activation: the leading cause of the prozone phenomenon affecting HLA antibody detection on single-antigen beads. Transplantation. 97:1279–85. DOI: 10.1097/01.TP.0000441091.47464.c6. PMID: 24621535.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of preexisting human leukocyte antigen donor-specific antibodies especially human leukocyte antigen-DQ on kidney transplant outcome
- Neonatal Alloimmune Thrombocytopenia Caused by Anti-HLA-A2 Alloantibodies Determined by Luminex Single Antigen Bead Assay
- Assessment of human leukocyte antigen antibody dynamics in patients awaiting kidney transplantation
- Causes of Positive Pretransplant Crossmatches in the Absence of Donor-Specific Anti-Human Leukocyte Antigen Antibodies: A Single-Center Experience
- Clinical significance of donor-specific anti-HLA-DR51/52/53 antibodies for antibody-mediated rejection in kidney transplant recipients