Lab Med Online.
2023 Oct;13(4):275-281. 10.47429/lmo.2023.13.4.275.
Overcoming Immunological Barriers in Xenotransplantation: A Historical Review of Previous Research and Future Directions
- Affiliations
-
- 1Department of Laboratory Medicine, Hallym University College of Medicine, Chuncheon, Korea
- KMID: 2552753
- DOI: http://doi.org/10.47429/lmo.2023.13.4.275
Abstract
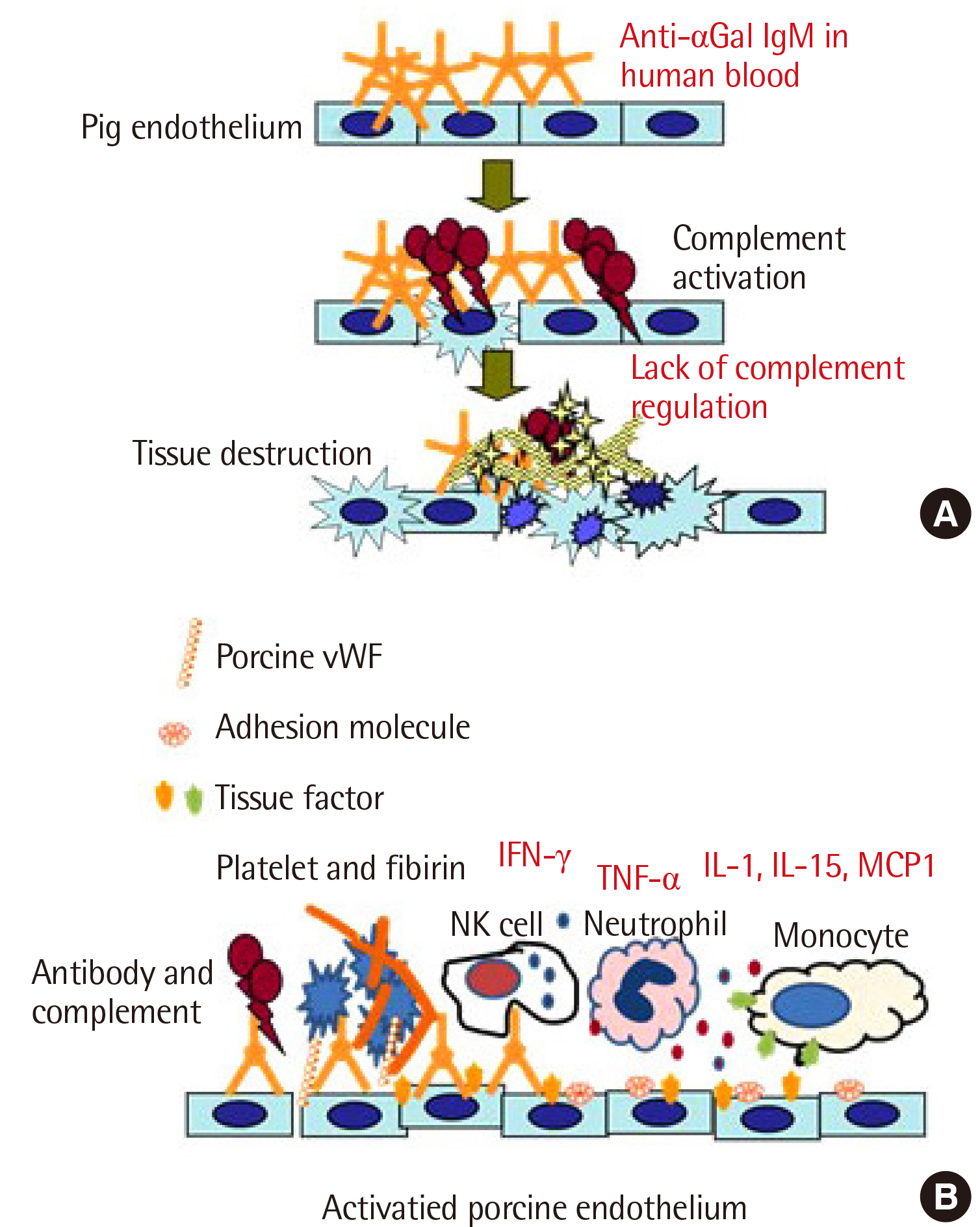
- The shortage of human donor organs is a major concern in clinical transplantation. Xenotransplantation, the transplantation of animal organs into humans, is a procedure that aims to solve this problem. Unfortunately, this ambitious idea has a long, fascinating, but disappointing history. Among the many obstacles to xenotransplantation, immunological barriers and molecular incompatibility between species are the most challenging. Recent advances in gene editing techniques and the development of targeted immune suppressive agents have pushed xenotransplantation closer to clinical application. In 2022, the first patient that received porcine heart transplantation in the United States showed no sign of hyperacute rejection. Although the patient did not survive for a long time, this controversial clinical trial proved that previous research data have been crucial in improving the viability of porcine donor organs in clinical transplantations. Xenotransplantation has shown a slight progress in its clinical application. However, further progress necessitates extensive and challenging research, but is worth pursuing. This article covers previous scientific endeavors and future directions in xenotransplantation research.
Figure
Reference
-
1. Korea Network for Organ Sharing. Annual report of the transplant 2019. https://www.konos.go.kr/board/boardListPage.do?page=sub4_2_1&boardId=30. Last accessed on Mar 2023.2. Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. 2012; Clinical xenotransplantation: the next medical revolution? Lancet. 379:672–83. DOI: 10.1016/S0140-6736(11)61091-X. PMID: 22019026.3. Griffith BP, Goerlich CE, Singh AK, Rothblatt M, Lau CL, Shah A, et al. 2022; Genetically modified porcine-to-human cardiac xenotransplantation. N Engl J Med. 387:35–44. DOI: 10.1056/NEJMoa2201422. PMID: 35731912. PMCID: PMC10361070.4. Cooper DKC, Ekser B, Tector AJ. 2015; A brief history of clinical xenotransplantation. Int J Surg. 23:205–10. DOI: 10.1016/j.ijsu.2015.06.060. PMID: 26118617. PMCID: PMC4684730.5. Aristizabal AM, Caicedo LA, Martínez JM, Moreno M, J Echeverri G. 2017; Clinical xenotransplantation, a closer reality: Literature review. Cir Esp. 95:62–72. DOI: 10.1016/j.ciresp.2016.12.008. PMID: 28237390.6. Cooper DK, Bottino R. 2015; Recent advances in understanding xenotransplantation: implications for the clinic. Expert Rev Clin Immunol. 11:1379–90. DOI: 10.1586/1744666X.2015.1083861. PMID: 26548357. PMCID: PMC4879962.7. Cozzi E, White DJ. 1995; The generation of transgenic pigs as potential organ donors for humans. Nat Med. 1:964–6. DOI: 10.1038/nm0995-964. PMID: 7585226.8. Rose AG, Cooper DK, Human PA, Reichenspurner H, Reichart B. 1991; Histopathology of hyperacute rejection of the heart: experimental and clinical observations in allografts and xenografts. J Heart Lung Transplant. 10:223–34.9. Najarian JS. 2003; Experimental xenotransplantation: a personal history. Xenotransplantation. 10:10–5. DOI: 10.1034/j.1399-3089.2003.01082.x. PMID: 12535221.10. Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, et al. 1992; Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 24:559–62.11. Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, et al. 1993; Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1:198–205. DOI: 10.1016/0966-3274(93)90047-C. PMID: 7521740.12. Huai G, Qi P, Yang H, Wang Y. 2016; Characteristics of α-Gal epitope, anti-Gal antibody, α1,3 galactosyltransferase and its clinical exploitation (Review). Int J Mol Med. 37:11–20. DOI: 10.3892/ijmm.2015.2397. PMID: 26531137. PMCID: PMC4687435.13. Galili U, Buehler J, Shohet SB, Macher BA. 1987; The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J Exp Med. 165:693–704. DOI: 10.1084/jem.165.3.693. PMID: 2434599. PMCID: PMC2188289.14. Galili U. 1993; Interaction of the natural anti-Gal antibody with α-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 14:480–2. DOI: 10.1016/0167-5699(93)90261-I. PMID: 7506033.15. Schuurman HJ, Pino-Chavez G, Phillips MJ, Thomas L, White DJ, Cozzi E. 2002; Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation. 73:1146–51. DOI: 10.1097/00007890-200204150-00024. PMID: 11965048.16. Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. 2009; Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 16:477–85. DOI: 10.1111/j.1399-3089.2009.00533.x. PMID: 20042047.17. Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. 2005; Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 11:29–31. DOI: 10.1038/nm1171. PMID: 15619628.18. Shimizu A, Hisashi Y, Kuwaki K, Tseng YL, Dor FJ, Houser SL, et al. 2008; Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am J Pathol. 172:1471–81. DOI: 10.2353/ajpath.2008.070672. PMID: 18467706. PMCID: PMC2408408.19. Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. 1996; Delayed xenograft rejection. Immunol Today. 17:379–84. DOI: 10.1016/0167-5699(96)10024-4. PMID: 8783499.20. Doudna JA, Charpentier E. 2014; The new frontier of genome engineering with CRISPR-Cas9. Science. 346:1258096. DOI: 10.1126/science.1258096. PMID: 25430774.21. Ma D, Hirose T, Lassiter G, Sasaki H, Rosales I, Coe TM, et al. 2022; Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am J Transplant. 22:46–57. DOI: 10.1111/ajt.16780. PMID: 34331749. PMCID: PMC9291868.22. Gaca JG, Lesher A, Aksoy O, Ruggeri ZM, Parker W, Davis RD. 2002; The role of the porcine von Willebrand factor: baboon platelet interactions in pulmonary xenotransplantation. Transplantation. 74:1596–603. DOI: 10.1097/00007890-200212150-00018. PMID: 12490794.23. Kang HJ, Lee G, Kim JY, Lee SH, Wi HC, Hwang PG, et al. 2005; Pre-treatment of donor with 1-deamino-8-d-arginine vasopressin could alleviate early failure of porcine xenograft in a cobra venom factor treated canine recipient. Eur J Cardiothorac Surg. 28:149–56. DOI: 10.1016/j.ejcts.2005.02.042. PMID: 15982598.24. Kim YT, Lee HJ, Lee SW, Kim JY, Wi HC, Park SJ, et al. 2008; Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 15:27–35. DOI: 10.1111/j.1399-3089.2008.00445.x. PMID: 18333911.25. Kang HJ, Lee H, Ha JM, Lee JI, Shin JS, Kim KY, et al. 2014; The role of the alternative complement pathway in early graft loss after intraportal porcine islet xenotransplantation. Transplantation. 97:999–1008. DOI: 10.1097/TP.0000000000000069. PMID: 24704666.26. Lee KG, Lee H, Ha JM, Lee YK, Kang HJ, Park CG, et al. 2012; Increased human tumor necrosis factor-α levels induce procoagulant change in porcine endothelial cells in vitro. Xenotransplantation. 19:186–95. DOI: 10.1111/j.1399-3089.2012.00704.x. PMID: 22702470.27. Kang HJ, Lee H, Park EM, Kim JM, Shin JS, Kim JS, et al. 2015; Dissociation between anti-porcine albumin and anti-Gal antibody responses in non-human primate recipients of intraportal porcine islet transplantation. Xenotransplantation. 22:124–34. DOI: 10.1111/xen.12152. PMID: 25641336.28. Shin JS, Kim JM, Kim JS, Min BH, Kim YH, Kim HJ, et al. 2015; Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 15:2837–50. DOI: 10.1111/ajt.13345. PMID: 26096041.29. Jung KC, Park CG, Jeon YK, Park HJ, Ban YL, Min HS, et al. 2011; In situ induction of dendritic cell-based T cell tolerance in humanized mice and nonhuman primates. J Exp Med. 208:2477–88. DOI: 10.1084/jem.20111242. PMID: 22025302. PMCID: PMC3256968.30. Shin JS, Min BH, Kim JM, Kim JS, Yoon IH, Kim HJ, et al. 2016; Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation. 23:300–9. DOI: 10.1111/xen.12246. PMID: 27387829.31. Kang HJ, Lee H, Park EM, Kim JM, Shin JS, Kim JS, et al. 2015; Increase in anti-Gal IgM level is associated with early graft failure in intraportal porcine islet xenotransplantation. Ann Lab Med. 35:611–7. DOI: 10.3343/alm.2015.35.6.611. PMID: 26354349. PMCID: PMC4579105.32. Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, et al. 2007; CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 115:1571–80. DOI: 10.1161/CIRCULATIONAHA.106.683201. PMID: 17372166.33. Choi HJ, Lee JJ, Kim DH, Kim MK, Lee HJ, Ko AY, et al. 2015; Blockade of CD40-CD154 costimulatory pathway promotes long-term survival of full-thickness porcine corneal grafts in nonhuman primates: clinically applicable xenocorneal transplantation. Am J Transplant. 15:628–41. DOI: 10.1111/ajt.13057. PMID: 25676390.34. Kim J, Choi SH, Lee HJ, Kim HP, Kang HJ, Kim JM, et al. 2018; Comparative efficacy of anti-CD40 antibody-mediated costimulation blockade on long-term survival of full-thickness porcine corneal grafts in nonhuman primates. Am J Transplant. 18:2330–41. DOI: 10.1111/ajt.14913. PMID: 29722120.35. Yoon CH, Choi SH, Choi HJ, Lee HJ, Kang HJ, Kim JM, et al. 2020; Long-term survival of full-thickness corneal xenografts from α1,3-galactosyltrans-ferase gene-knockout miniature pigs in non-human primates. Xenotransplantation. 27:e12559. DOI: 10.1111/xen.12559. PMID: 31566261.36. Lee SJ, Kim JS, Chee HK, Yun IJ, Park KS, Yang HS, et al. 2018; Seven years of experiences of preclinical experiments of xeno-heart transplantation of pig to non-human primate (cynomolgus monkey). Transplant Proc. 50:1167–71. DOI: 10.1016/j.transproceed.2018.01.041. PMID: 29731087.37. Park EM, Lee H, Kang HJ, Oh KB, Kim JS, Chee HK, et al. 2021; Early interferon-gamma response in nonhuman primate recipients of solid-organ xenotransplantation. Transplant Proc. 53:3093–100. DOI: 10.1016/j.transproceed.2021.09.028. PMID: 34763883.38. Fishman JA. 2022; Risks of infectious disease in xenotransplantation. N Engl J Med. 387:2258–67. DOI: 10.1056/NEJMra2207462. PMID: 36516091.39. Denner J, Längin M, Reichart B, Krüger L, Fiebig U, Mokelke M, et al. 2020; Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci Rep. 10:17531. DOI: 10.1038/s41598-020-73150-9. PMID: 33067513. PMCID: PMC7568528.40. Ariyoshi Y, Takeuchi K, Pomposelli T, Ekanayake-Alper DK, Shimizu A, Boyd L, et al. 2021; Antibody reactivity with new antigens revealed in multi-transgenic triple knockout pigs may cause early loss of pig kidneys in baboons. Xenotransplantation. 28:e12642. DOI: 10.1111/xen.12642. PMID: 32909301. PMCID: PMC8957702.41. Phimister EG. 2022; Genetic modification in pig-to-human transplantation. N Engl J Med. 387:79–82. DOI: 10.1056/NEJMe2207422. PMID: 35731913.42. Watanabe H, Ariyoshi Y, Pomposelli T, Takeuchi K, Ekanayake-Alper DK, Boyd LK, et al. 2020; Intra-bone bone marrow transplantation from hCD47 transgenic pigs to baboons prolongs chimerism to >60 days and promotes increased porcine lung transplant survival. Xenotransplantation. 27:e12552. DOI: 10.1111/xen.12552. PMID: 31544995. PMCID: PMC7007336.43. Shah JA, Patel MS, Elias N, Navarro-Alvarez N, Rosales I, Wilkinson RA, et al. 2017; Prolonged survival following pig-to-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade. Am J Transplant. 17:2178–85. DOI: 10.1111/ajt.14341. PMID: 28489305. PMCID: PMC5519420.44. Kim SC, Mathews DV, Breeden CP, Higginbotham LB, Ladowski J, Martens G, et al. 2019; Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am J Transplant. 19:2174–85. DOI: 10.1111/ajt.15329. PMID: 30821922. PMCID: PMC6658347.45. Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, et al. 2018; Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 564:430–3. DOI: 10.1038/s41586-018-0765-z. PMID: 30518863.