Diabetes Metab J.
2024 Jan;48(1):59-71. 10.4093/dmj.2022.0292.
A New Concept in Antidiabetic Therapeutics: A Concerted Removal of Labile Iron and Intracellular Deposition of Zinc
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, Institute of Medical Research Israel-Canada, The Hebrew University of Jerusalem, Jerusalem (HUJI), Jerusalem, Israel
- 2Concenter Biopharma, Jerusalem, Israel
- KMID: 2551263
- DOI: http://doi.org/10.4093/dmj.2022.0292
Abstract
- Background
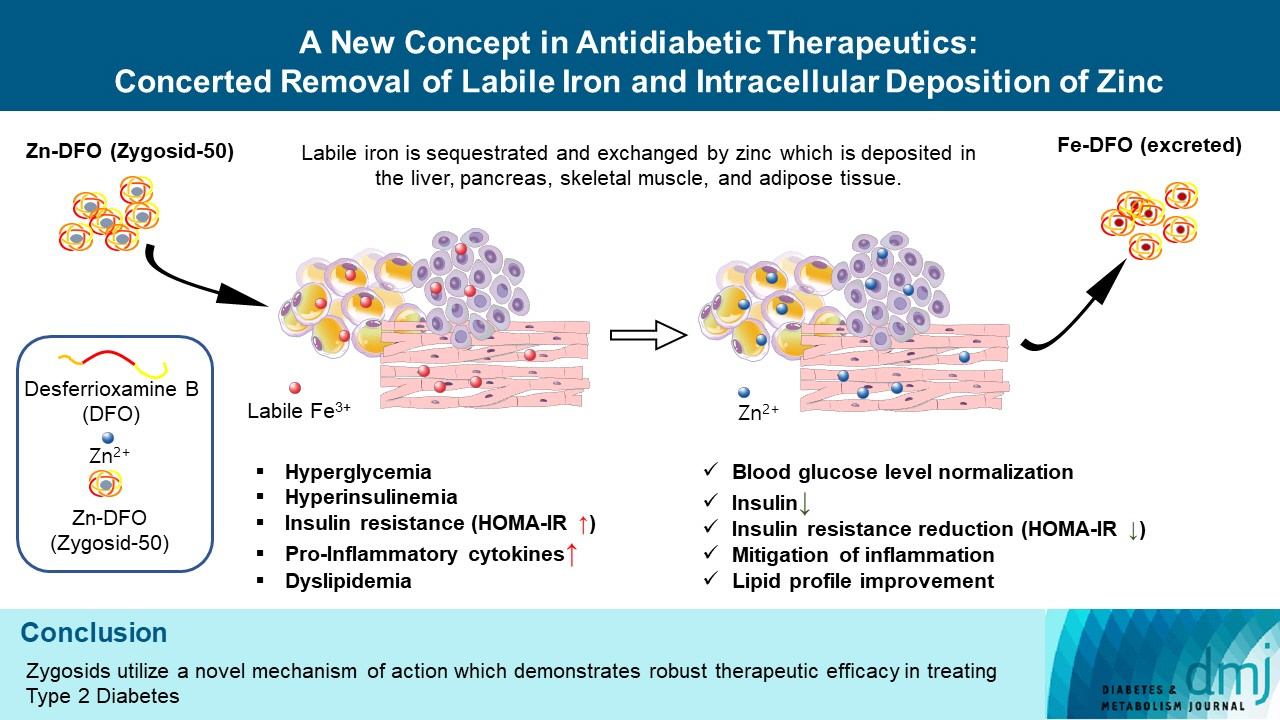
The inflammatory process is known to be an integral part of the pathophysiology of type 2 diabetes mellitus (T2DM). The “labile,” redox-active iron, serving as a catalyst in Fenton reaction, producing the deleterious reactive oxygen species, triggering and maintaining inflammation, is hypothesized to play a causative role in this process. Concenter Biopharma continued the development of a new platform of iron chelators (Zygosids), first initiated at the Hebrew University of Jerusalem, Israel (HUJI), acting via the novel mechanism, based on a sequestration of the labile redox-active iron and its substitution by zinc or gallium. The mode of action of Zygosids is based on the higher affinity of the metal-binding moiety of the complex to Fe3+ in comparison to already bound ion, leading to rapid release of the ion of another metal and chelation of Fe3+. Concomitantly, zinc ion, released by the complex, is known for its antidiabetic and anti-inflammatory role.
Methods
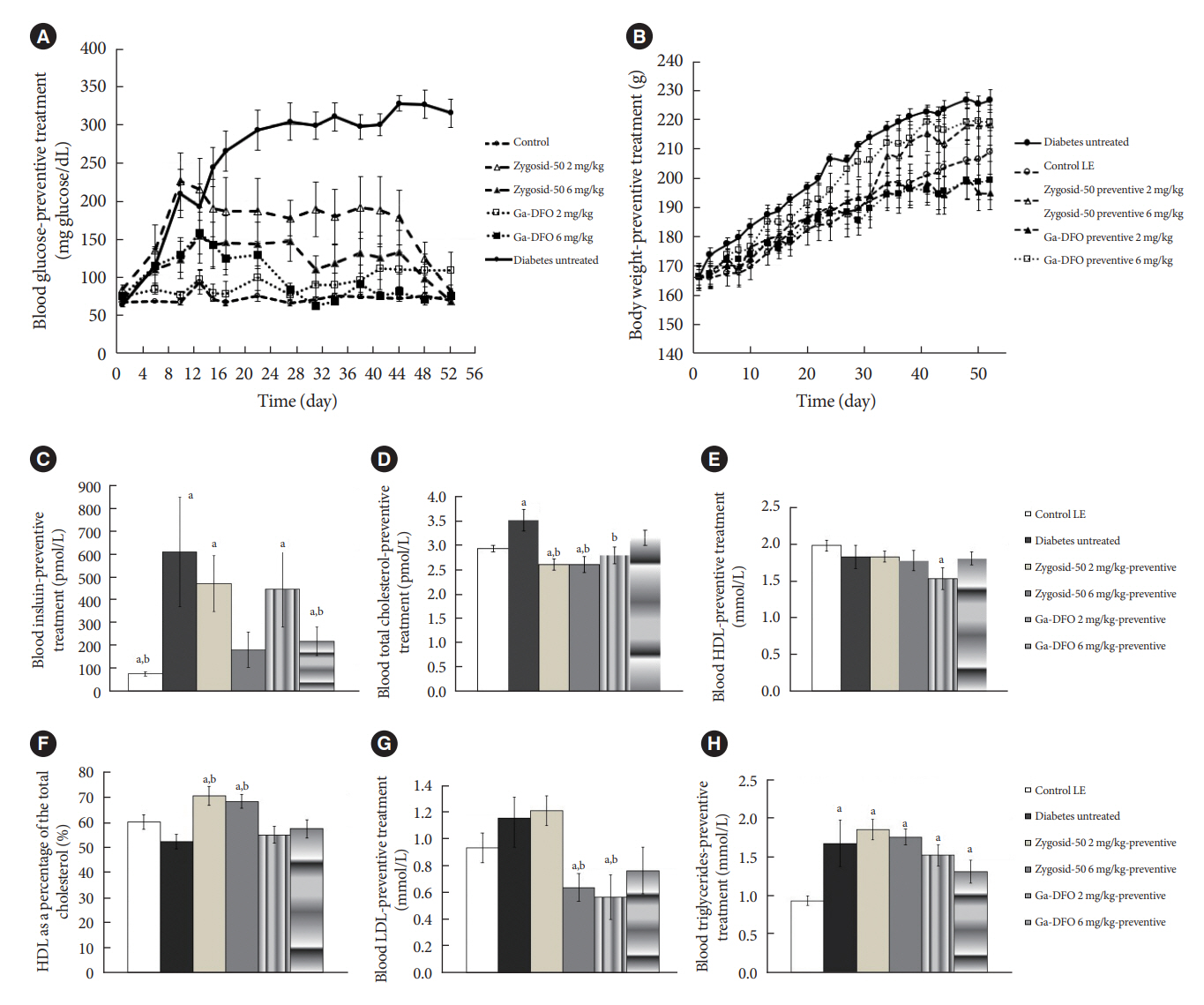
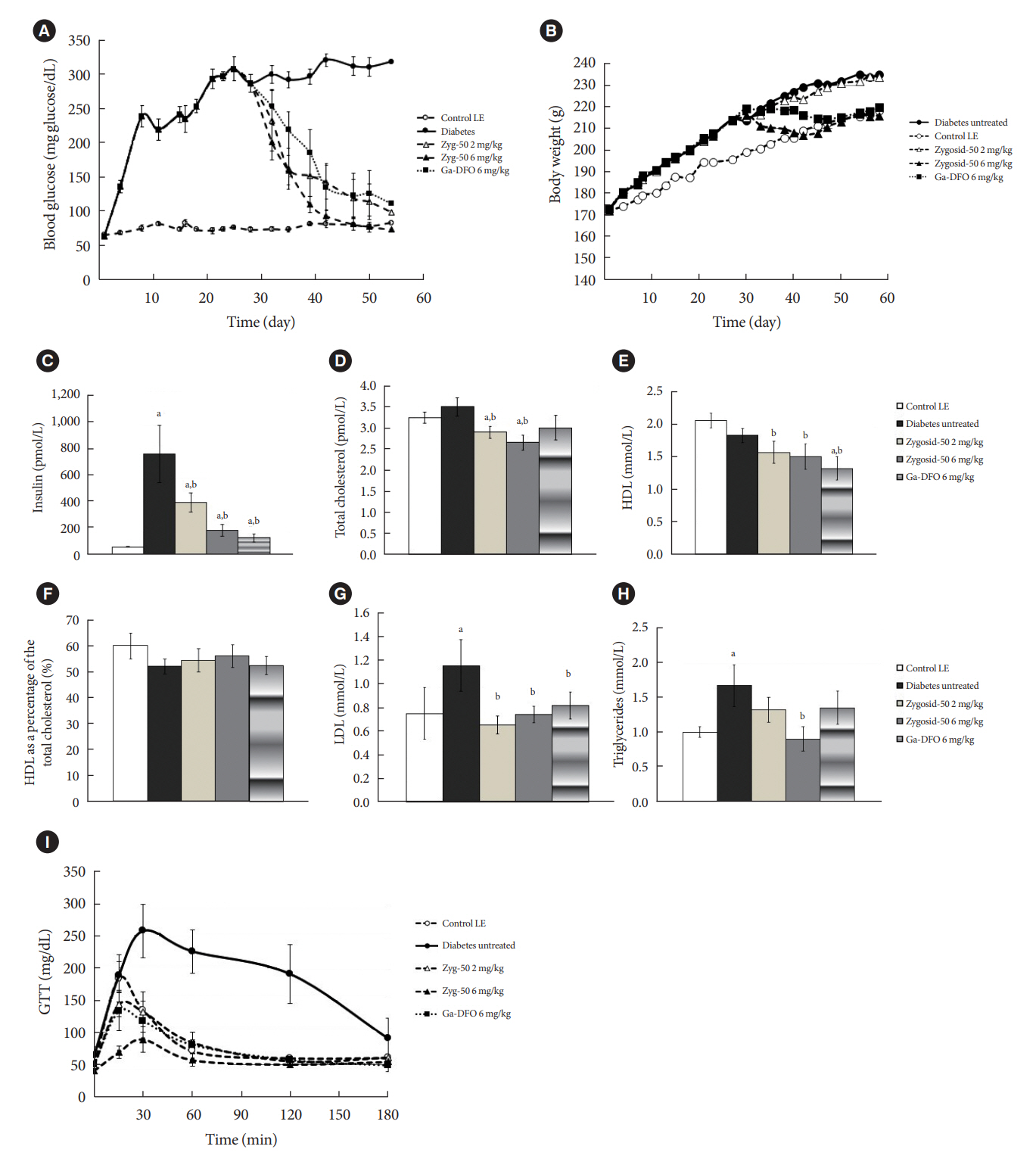
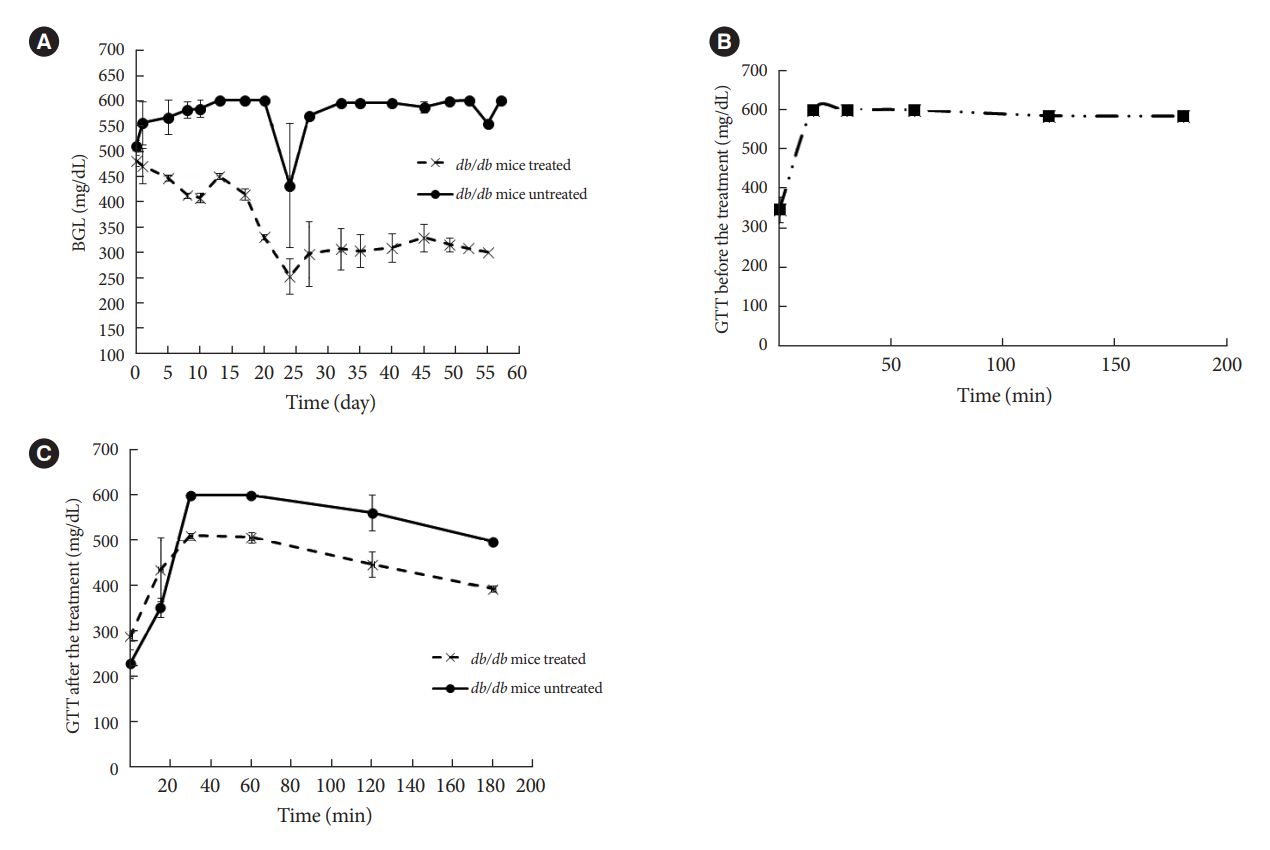
The therapeutic effect of zinc-desferrioxamine (Zygosid-50) and gallium-desferrioxamine, was tested on fat sand rat (Psammomys obesus) model of diet-induced T2DM and on Leprdb transgenic diabetic mice.
Results
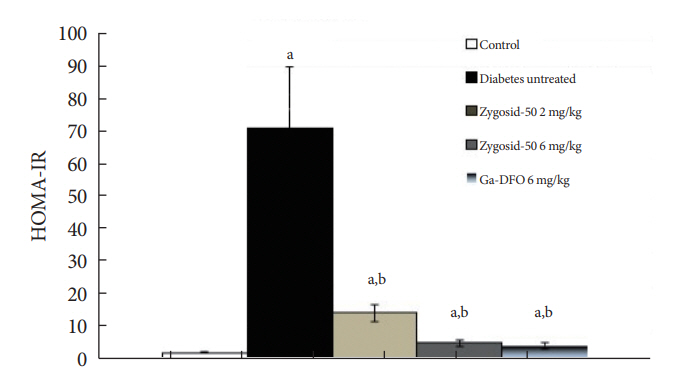
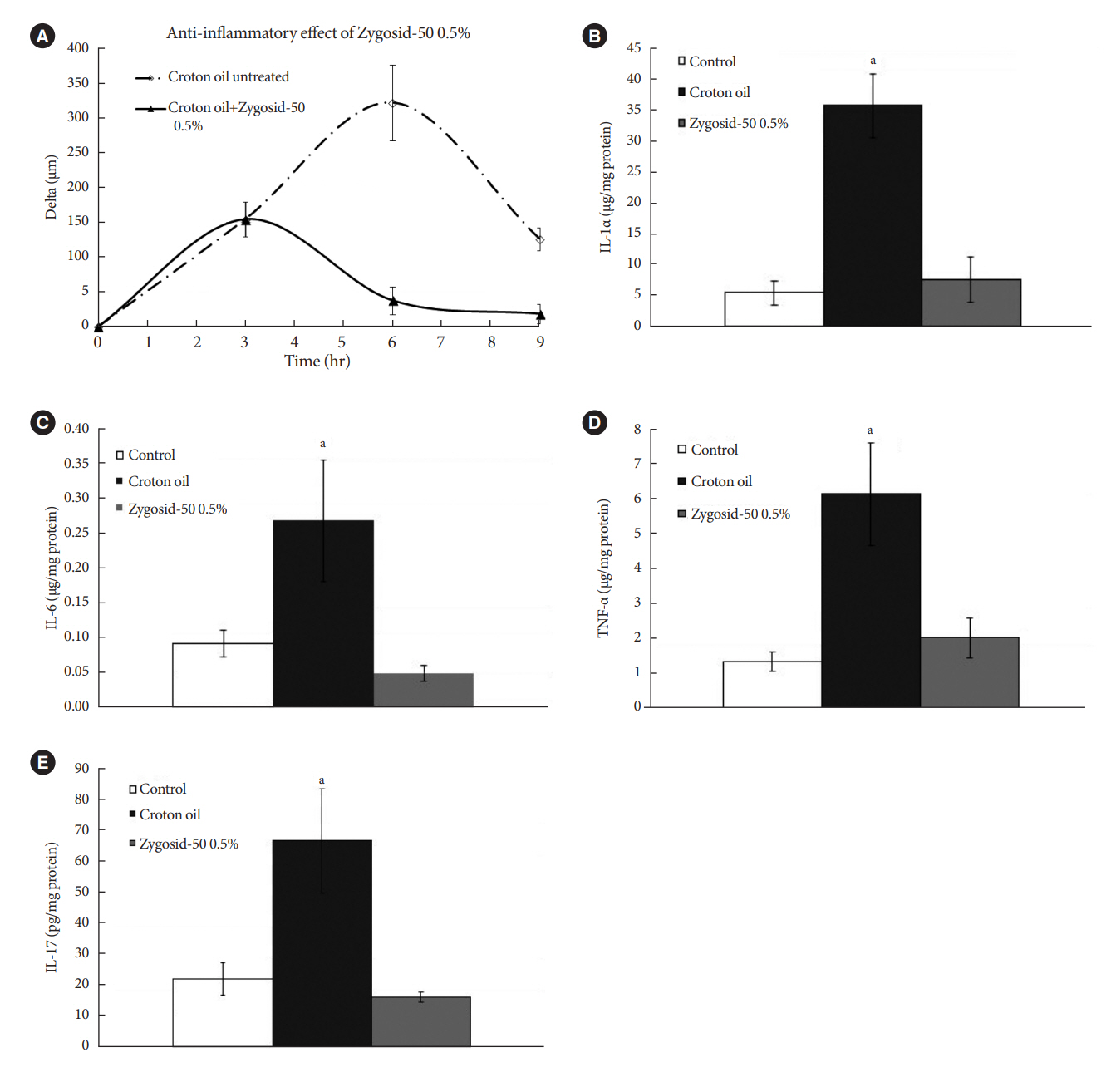
Zygosids demonstrated an ability to noticeably reduce blood glucose and insulin levels and improve the lipid profile. Moreover, an ability to mitigate insulin resistance by >90% was shown on the sand rat model. In addition, a potent anti-inflammatory effect, expressed as a diminishment of the proinflammatory cytokines in tissue levels, was demonstrated.
Conclusion
Zygosids demonstrated robust therapeutic efficacy in treatment of T2DM. Importantly, no adverse effects were detected, in all the experiments, indicating high safety profile.
Figure
Reference
-
1. O’Connell JM, Manson SM. Understanding the economic costs of diabetes and prediabetes and what we may learn about reducing the health and economic burden of these conditions. Diabetes Care. 2019; 42:1609–11.2. GlobalData. Type 2 diabetes market to more than double, to $64 billion by 2026. Available from: https://www.globaldata.com/media/press-release/type-2-diabetes-market-double-64-billion-2026 (cited 2023 May 15).3. Fernandez-Real JM, McClain D, Manco M. Mechanisms linking glucose homeostasis and iron metabolism toward the onset and progression of type 2 diabetes. Diabetes Care. 2015; 38:2169–76.4. Chirumbolo S, Rossi AP, Rizzatti V, Zoico E, Franceschetti G, Girelli D, et al. Iron primes 3T3-L1 adipocytes to a TLR4-mediated inflammatory response. Nutrition. 2015; 31:1266–74.5. Andrews M, Soto N, Arredondo-Olguin M. Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition. 2015; 31:51–7.6. MacDonald MJ, Cook JD, Epstein ML, Flowers CH. Large amount of (apo)ferritin in the pancreatic insulin cell and its stimulation by glucose. FASEB J. 1994; 8:777–81.7. Aigner E, Felder TK, Oberkofler H, Hahne P, Auer S, Soyal S, et al. Glucose acts as a regulator of serum iron by increasing serum hepcidin concentrations. J Nutr Biochem. 2013; 24:112–7.8. Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, et al. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia. 1984; 26:441–4.9. Davis RJ, Corvera S, Czech MP. Insulin stimulates cellular iron uptake and causes the redistribution of intracellular transferrin receptors to the plasma membrane. J Biol Chem. 1986; 261:8708–11.10. Ferrannini E. Insulin resistance, iron, and the liver. Lancet. 2000; 355:2181–2.11. Dongiovanni P, Ruscica M, Rametta R, Recalcati S, Steffani L, Gatti S, et al. Dietary iron overload induces visceral adipose tissue insulin resistance. Am J Pathol. 2013; 182:2254–63.12. Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002; 51:2348–54.13. Cutler P. Deferoxamine therapy in high-ferritin diabetes. Diabetes. 1989; 38:1207–10.14. Kaye TB, Guay AT, Simonson DC. Non-insulin-dependent diabetes mellitus and elevated serum ferritin level. J Diabetes Complications. 1993; 7:246–9.15. De Sanctis V, Soliman A, Yassin M. Iron overload and glucose metabolism in subjects with β-thalassaemia major: an overview. Curr Diabetes Rev. 2013; 9:332–41.16. Chevion M. Protection against free radical-induced and transition metal-mediated damage: the use of “pull” and “push” mechanisms. Free Radic Res Commun. 1991; 12-13 Pt 2:691–6.17. Keberle H. The biochemistry of desferrioxamine and its relation to iron metabolism. Ann N Y Acad Sci. 1964; 119:758–68.18. Hjorth CF, Norrman M, Wahlund PO, Benie AJ, Petersen BO, Jessen CM, et al. Structure, aggregation, and activity of a covalent insulin dimer formed during storage of neutral formulation of human insulin. J Pharm Sci. 2016; 105:1376–86.19. Egefjord L, Petersen AB, Rungby J. Zinc, alpha cells and glucagon secretion. Curr Diabetes Rev. 2010; 6:52–7.20. Norouzi S, Adulcikas J, Sohal SS, Myers S. Zinc transporters and insulin resistance: therapeutic implications for type 2 diabetes and metabolic disease. J Biomed Sci. 2017; 24:87.21. Wu Y, Lu H, Yang H, Li C, Sang Q, Liu X, et al. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: essential roles of Akt-GLUT4, GSK3β and mTOR-S6K1. J Nutr Biochem. 2016; 34:126–35.22. Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. 2018; 68:19–31.23. Jansen J, Rosenkranz E, Overbeck S, Warmuth S, Mocchegiani E, Giacconi R, et al. Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. J Nutr Biochem. 2012; 23:1458–66.24. Kaiser N, Cerasi E, Leibowitz G. Diet-induced diabetes in the sand rat (Psammomys obesus). Methods Mol Biol. 2012; 933:89–102.25. Bibi H, Vinokur V, Waisman D, Elenberg Y, Landesberg A, Faingersh A, et al. Zn/Ga-DFO iron-chelating complex attenuates the inflammatory process in a mouse model of asthma. Redox Biol. 2014; 2:814–9.26. Anis Y, Leshem O, Reuveni H, Wexler I, Ben Sasson R, Yahalom B, et al. Antidiabetic effect of novel modulating peptides of G-protein-coupled kinase in experimental models of diabetes. Diabetologia. 2004; 47:1232–44.27. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999; 94:2467–74.28. Goebeler M, Gutwald J, Roth J, Sorg C. The severity of irritant contact dermatitis in various strains of mice correlates with endothelial expression of migration inhibitory factor (MIF). Arch Dermatol Res. 1991; 283:246–50.29. Ryan BJ, Van Pelt DW, Guth LM, Ludzki AC, Gioscia-Ryan RA, Ahn C, et al. Plasma ferritin concentration is positively associated with in vivo fatty acid mobilization and insulin resistance in obese women. Exp Physiol. 2018; 103:1443–7.30. Liu P, Gan W, Inuzuka H, Lazorchak AS, Gao D, Arojo O, et al. Sin1 phosphorylation impairs mTORC2 complex integrity and inhibits downstream Akt signalling to suppress tumorigenesis. Nat Cell Biol. 2013; 15:1340–50.31. Cui R, Choi SE, Kim TH, Lee HJ, Lee SJ, Kang Y, et al. Iron overload by transferrin receptor protein 1 regulation plays an important role in palmitate-induced insulin resistance in human skeletal muscle cells. FASEB J. 2019; 33:1771–86.32. Koenig G, Seneff S. Gamma-glutamyltransferase: a predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers. 2015; 2015:818570.33. Sun L, Qi Q, Zong G, Ye X, Li H, Liu X, et al. Elevated plasma retinol-binding protein 4 is associated with increased risk of type 2 diabetes in middle-aged and elderly Chinese adults. J Nutr. 2014; 144:722–8.34. Juanola-Falgarona M, Candido-Fernandez J, Salas-Salvado J, Martinez-Gonzalez MA, Estruch R, Fiol M, et al. Association between serum ferritin and osteocalcin as a potential mechanism explaining the iron-induced insulin resistance. PLoS One. 2013; 8:e76433.35. Wieser V, Moschen AR, Tilg H. Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp (Warsz). 2013; 61:119–25.36. Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Basfi-fer K, et al. Zinc as a potential coadjuvant in therapy for type 2 diabetes. Food Nutr Bull. 2013; 34:215–21.37. Prasad AS. Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr. 2014; 1:14.38. Luo YY, Zhao J, Han XY, Zhou XH, Wu J, Ji LN. Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin Med J (Engl). 2015; 128:3276–82.39. Chieppa M, Galleggiante V, Serino G, Massaro M, Santino A. Iron chelators dictate immune cells inflammatory ability: potential adjuvant therapy for IBD. Curr Pharm Des. 2017; 23:2289–98.40. Vinokur V, Berenshtein E, Chevion MM, Eliashar R. Iron homeostasis and methionine-centred redox cycle in nasal polyposis. Free Radic Res. 2011; 45:366–73.41. Phillipson OT. Management of the aging risk factor for Parkinson’s disease. Neurobiol Aging. 2014; 35:847–57.42. Martins AC, Almeida JI, Lima IS, Kapitao AS, Gozzelino R. Iron metabolism and the inflammatory response. IUBMB Life. 2017; 69:442–50.43. Wong CP, Rinaldi NA, Ho E. Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res. 2015; 59:991–9.44. Li HT, Jiao M, Chen J, Liang Y. Roles of zinc and copper in modulating the oxidative refolding of bovine copper, zinc superoxide dismutase. Acta Biochim Biophys Sin (Shanghai). 2010; 42:183–94.45. Banin E, Berenshtein E, Kitrossky N, Pe’er J, Chevion M. Gallium-desferrioxamine protects the cat retina against injury after ischemia and reperfusion. Free Radic Biol Med. 2000; 28:315–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A New Concept in Antidiabetic Therapeutics: A Concerted Removal of Labile Iron and Intracellular Deposition of Zinc
- Concentrations of lead, iron and zinc in blood of coal workers' pneumoconiosis patients
- HDL cholesterol, copper, ceruloplasmin, zinc, iron values of the blood in newborn
- Therapeutic Iron and Zinc Supplementation in Children
- A Study on the Balance of Iron and Zinc in Korean Children