Ewha Med J.
2023 Dec;46(S1):e25. 10.12771/emj.2023.e25.
How Can We Improve the Tumor Response to Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer?
- Affiliations
-
- 1Department of Surgery, Division of Colorectal Surgery, CHA Bundang Medical Center, Seongnam, Korea
- KMID: 2550845
- DOI: http://doi.org/10.12771/emj.2023.e25
Abstract
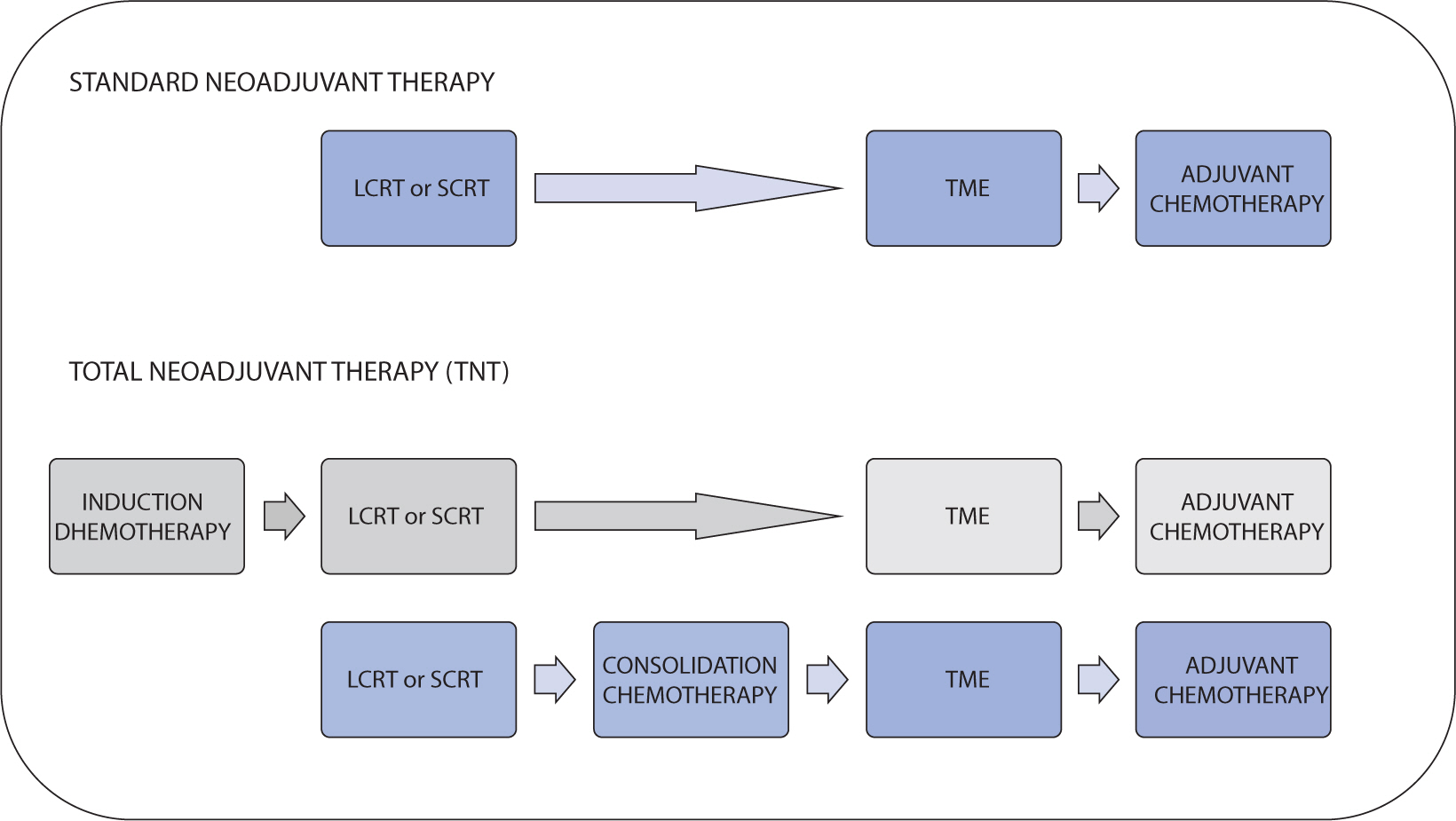
- Preoperative chemoradiotherapy (pCRT) followed by total mesorectal excision is the accepted standard treatment for patients with locally advanced rectal cancer. The purpose of pCRT is to prevent the spread of viable tumor cells within the local area during surgical procedures. Additionally, pCRT can facilitate the resection of locally advanced tumors that are otherwise challenging to remove, thereby enabling a radical resection. Although a pathologic complete response is observed in fewer than 20% of patients, the reasons for the variability in tumor response to pCRT are not fully understood. Several techniques have been researched with the aim of improving the tumor response to pCRT. These techniques include intensifying or combining chemotherapy, either simultaneously or sequentially, increasing radiation dose, modifying radiation mode or schedule, adjusting the interval between radiation and surgery, and incorporating multiple agents to increase the efficacy of pCRT. This review discusses various strategies that may improve tumor response outcomes following pCRT.
Figure
Reference
-
References
1. Kim MH, Park S, Yi N, Kang B, Park IJ. Colorectal cancer mortality trends in the era of cancer survivorship in Korea: 2000–2020. Ann Coloproctol. 2022; 38(5):343–352. DOI: 10.3393/ac.2022.00535.0076. PMID: 36353833. PMCID: PMC9650346.2. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004; 351(17):1731–1740. DOI: 10.1056/NEJMoa040694. PMID: 15496622.3. Varela C, Kim NK. Surgical treatment of low-lying rectal cancer: updates. Ann Coloproctol. 2021; 37(6):395–424. DOI: 10.3393/ac.2021.00927.0132. PMID: 34961303. PMCID: PMC8717072.4. Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009; 250(4):582–589. DOI: 10.1097/SLA.0b013e3181b91e63. PMID: 19710605.5. Dattani M, Heald RJ, Goussous G, Broadhurst J, São Julião GP, Habr-Gama A, et al. Oncological and survival outcomes in watch and wait patients with a clinical complete response after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and pooled analysis. Ann Surg. 2018; 268(6):955–967. DOI: 10.1097/SLA.0000000000002761. PMID: 29746338.6. Park IJ. Precision medicine for primary rectal cancer will become a reality. Ann Coloproctol. 2022; 38(4):281–282. DOI: 10.3393/ac.2022.00500.0071. PMID: 36059074. PMCID: PMC9441542.7. An SH, Kim IY. Can pretreatment platelet-to-lymphocyte and neutrophil-to-lymphocyte ratios predict long-term oncologic outcomes after preoperative chemoradiation followed by surgery for locally advanced rectal cancer? Ann Coloproctol. 2022; 38(3):253–261. DOI: 10.3393/ac.2021.00633.0090. PMID: 35249276. PMCID: PMC9263313.8. Appelt AL, Pløen J, Vogelius IR, Bentzen SM, Jakobsen A. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013; 85(1):74–80. DOI: 10.1016/j.ijrobp.2012.05.017. PMID: 22763027. PMCID: PMC3539741.9. Burbach JPM, den Harder AM, Intven M, Van Vulpen M, Verkooijen HM, Reerink O. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol. 2014; 113(1):1–9. DOI: 10.1016/j.radonc.2014.08.035. PMID: 25281582.10. Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: a randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012; 84(4):949–954. DOI: 10.1016/j.ijrobp.2012.02.006. PMID: 22592048.11. Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012; 30(36):4558–4565. DOI: 10.1200/JCO.2012.42.8771. PMID: 23109696.12. Akce M, El-Rayes BF. Nonsurgical management of rectal cancer. J Oncol Pract. 2019; 15(3):123–131. DOI: 10.1200/JOP.18.00769. PMID: 30861368.13. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2017; 28(suppl_4):iv22–iv40. DOI: 10.1093/annonc/mdx224. PMID: 28881920.14. Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010; 53(12):1692–1698. DOI: 10.1007/DCR.0b013e3181f42b89. PMID: 21178866.15. De Palma FDE, Luglio G, Tropeano FP, Pagano G, D'Armiento M, Kroemer G, et al. The role of micro-RNAs and circulating tumor markers as predictors of response to neoadjuvant therapy in locally advanced rectal cancer. Int J Mol Sci. 2020; 21(19):7040. DOI: 10.3390/ijms21197040. PMID: 32987896. PMCID: PMC7582560.16. Trakarnsanga A, Gönen M, Shia J, Nash GM, Temple LK, Guillem JG, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014; 106(10):dju248. DOI: 10.1093/jnci/dju248. PMID: 25249540. PMCID: PMC4271114.17. Gani C, Kirschniak A, Zips D. Watchful waiting after radiochemotherapy in rectal cancer: when is it feasible? Visc Med. 2019; 35(2):119–123. DOI: 10.1159/000499167. PMID: 31192245. PMCID: PMC6514498.18. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Pudełko M, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004; 72(1):15–24. DOI: 10.1016/j.radonc.2003.12.006. PMID: 15236870.19. Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012; 30(31):3827–3833. DOI: 10.1200/JCO.2012.42.9597. PMID: 23008301.20. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006; 93(10):1215–1223. DOI: 10.1002/bjs.5506. PMID: 16983741.21. Pettersson D, Cedermark B, Holm T, Radu C, Påhlman L, Glimelius B, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg. 2010; 97(4):580–587. DOI: 10.1002/bjs.6914. PMID: 20155787.22. Pettersson D, Holm T, Iversen H, Blomqvist L, Glimelius B, Martling A. Preoperative short-course radiotherapy with delayed surgery in primary rectal cancer. Br J Surg. 2012; 99(4):577–583. DOI: 10.1002/bjs.7796. PMID: 22241246.23. Park KJ. Against all odds: why surgeons need to be more aggressive in the era of the multidisciplinary team approach to colorectal cancer. Ann Coloproctol. 2022; 38(6):393–397. DOI: 10.3393/ac.2022.00822.0117. PMID: 36596299. PMCID: PMC9816559.24. Bernier L, Balyasnikova S, Tait D, Brown G. Watch-and-wait as a therapeutic strategy in rectal cancer. Curr Colorectal Cancer Rep. 2018; 14(2):37–55. DOI: 10.1007/s11888-018-0398-5. PMID: 29576755. PMCID: PMC5857277.25. Marijnen CA, Nagtegaal ID, Klein Kranenbarg E, Hermans J, De Velde CJ, Leer JW, et al. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001; 19(7):1976–1984. DOI: 10.1200/JCO.2001.19.7.1976. PMID: 11283130.26. Pettersson D, Lörinc E, Holm T, Iversen H, Cedermark B, Glimelius B, et al. Tumour regression in the randomized Stockholm III trial of radiotherapy regimens for rectal cancer. Br J Surg. 2015; 102(8):972–978. DOI: 10.1002/bjs.9811. PMID: 26095256. PMCID: PMC4744683.27. Garcia-Aguilar J, Patil S, Gollub MJ, Kim JK, Yuval JB, Thompson HM, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022; 40(23):2546–2556. DOI: 10.1200/JCO.22.00032. PMID: 35483010. PMCID: PMC9362876.28. Guzmán Y, Ríos J, Paredes J, Domínguez P, Maurel J, González-Abós C, et al. Time interval between the end of neoadjuvant therapy and elective resection of locally advanced rectal cancer in the CRONOS study. JAMA Surg. 2023; 158(9):910–919. DOI: 10.1001/jamasurg.2023.2521. PMID: 37436726.29. Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999; 17(8):2396. DOI: 10.1200/JCO.1999.17.8.2396. PMID: 10561302.30. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, De Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol. 2016; 34(31):3773–3780. DOI: 10.1200/JCO.2016.67.6049. PMID: 27432930.31. Sun Z, Adam MA, Kim J, Shenoi M, Migaly J, Mantyh CR. Optimal timing to surgery after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Am Coll Surg. 2016; 222(4):367–374. DOI: 10.1016/j.jamcollsurg.2015.12.017. PMID: 26897480.32. Du D, Su Z, Wang D, Liu W, Wei Z. Optimal interval to surgery after neoadjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2018; 17(1):13–24. DOI: 10.1016/j.clcc.2017.10.012. PMID: 29153429.33. Figueiredo N, Panteleimonitis S, Popeskou S, Cunha JF, Qureshi T, Beets GL, et al. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J Surg Oncol. 2018; 44(4):484–489. DOI: 10.1016/j.ejso.2018.01.088. PMID: 29398323.34. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015; 16(8):957–966. DOI: 10.1016/S1470-2045(15)00004-2. PMID: 26187751.35. Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012; 13(7):679–687. DOI: 10.1016/S1470-2045(12)70187-0. PMID: 22627104.36. Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015; 16(8):979–989. DOI: 10.1016/S1470-2045(15)00159-X. PMID: 26189067.37. Zhu J, Liu A, Sun X, Liu L, Zhu Y, Zhang T, et al. Multicenter, randomized, phase III trial of neoadjuvant chemoradiation with capecitabine and irinotecan guided by UGT1A1 status in patients with locally advanced rectal cancer. J Clin Oncol. 2020; 38(36):4231–4239. DOI: 10.1200/JCO.20.01932. PMID: 33119477. PMCID: PMC7768334.38. Gollins S, Myint AS, Haylock B, Wise M, Saunders M, Neupane R, et al. Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcomes. J Clin Oncol. 2011; 29(8):1042–1049. DOI: 10.1200/JCO.2010.29.7697. PMID: 21263095.39. Mohiuddin M, Paulus R, Mitchell E, Hanna N, Yuen A, Nichols R, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. Int J Radiat Oncol Biol Phys. 2013; 86(3):523–528. DOI: 10.1016/j.ijrobp.2013.02.020. PMID: 23545284. PMCID: PMC4201041.40. Hospers G, Bahadoer RR, Dijkstra EA, Van Etten B, Marijnen C, Putter H, et al. Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: the randomized RAPIDO trial. J Clin Oncol. 2020; 38((15 Suppl)):4006. DOI: 10.1200/JCO.2020.38.15_suppl.4006.41. Dijkstra EA, Nilsson PJ, Hospers GAP, Bahadoer RR, Elma MKK, Annet R, et al. Locoregional failure during and after short-course radiotherapy followed by chemotherapy and surgery compared to long-course chemoradiotherapy and surgery: a 5-year follow-up of the RAPIDO trial. Ann Surg. 2023; 278(4):e766–e772. DOI: 10.1097/SLA.0000000000005799. PMID: 36661037. PMCID: PMC10481913.42. Conroy T, Lamfichekh N, Etienne PL, Rio E, Francois E, Mesgouez-Nebout N, et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. J Clin Oncol. 2020; 38((15 Suppl)):4007. DOI: 10.1200/JCO.2020.38.15_suppl.4007.43. Resch G, De Vries A, Öfner D, Eisterer W, Rabl H, Jagoditsch M, et al. Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer: a two stage phase II clinical trial. Radiother Oncol. 2012; 102(1):10–13. DOI: 10.1016/j.radonc.2011.06.008. PMID: 21741716.44. Kennecke H, Berry S, Wong R, Zhou C, Tankel K, Easaw J, et al. Pre-operative bevacizumab, capecitabine, oxaliplatin and radiation among patients with locally advanced or low rectal cancer: a phase II trial. Eur J Cancer. 2012; 48(1):37–45. DOI: 10.1016/j.ejca.2011.05.016. PMID: 21664123.45. Sclafani F, Gonzalez D, Cunningham D, Hulkki Wilson S, Peckitt C, Giralt J, et al. RAS mutations and cetuximab in locally advanced rectal cancer: results of the EXPERT-C trial. Eur J Cancer. 2014; 50(8):1430–1436. DOI: 10.1016/j.ejca.2014.02.002. PMID: 24582914.46. Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004; 10(2):145–147. DOI: 10.1038/nm988. PMID: 14745444. PMCID: PMC2693485.47. Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009; 27(18):3020–3026. DOI: 10.1200/JCO.2008.21.1771. PMID: 19470921. PMCID: PMC2702234.48. Czito BG, Bendell JC, Willett CG, Morse MA, Blobe GC, Tyler DS, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: phase I trial results. Int J Radiat Oncol Biol Phys. 2007; 68(2):472–478. DOI: 10.1016/j.ijrobp.2007.02.001. PMID: 17498568.49. Clifford R, Govindarajah N, Parsons JL, Gollins S, West NP, Vimalachandran D. Systematic review of treatment intensification using novel agents for chemoradiotherapy in rectal cancer. Br J Surg. 2018; 105(12):1553–1572. DOI: 10.1002/bjs.10993. PMID: 30311641. PMCID: PMC6282533.50. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022; 386(25):2363–2376. DOI: 10.1056/NEJMoa2201445. PMID: 35660797. PMCID: PMC9492301.51. Grassi E, Zingaretti C, Petracci E, Corbelli J, Papiani G, Banchelli I, et al. Phase II study of capecitabine-based concomitant chemoradiation followed by durvalumab as a neoadjuvant strategy in locally advanced rectal cancer: the PANDORA trial. ESMO Open. 2023; 8(5):101824. DOI: 10.1016/j.esmoop.2023.101824. PMID: 37774508. PMCID: PMC10594026.52. Yuki S, Bando H, Tsukada Y, Inamori K, Komatsu Y, Homma S, et al. Short-term results of VOLTAGE-A: nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. J Clin Oncol. 2020; 38((15 Suppl)):4100. DOI: 10.1200/JCO.2020.38.15_suppl.4100.53. George TJ, Yothers G, Jacobs SA, Finley GG, Parekh HD, Moore TD, et al. NSABP FR-2: phase II study of durvalumab following neoadjuvant chemoRT in stage II-IV rectal cancer. J Clin Oncol. 2020; 38((4 Suppl)):TPS264. DOI: 10.1200/JCO.2020.38.4_suppl.TPS264.54. Ng JY, Chen CC. Transanal total mesorectal excision for rectal cancer: it’s come a long way and here to stay. Ann Coloproctol. 2022; 38(4):283–289. DOI: 10.3393/ac.2022.00374.0053. PMID: 36059075. PMCID: PMC9441544.55. Nasir IUI, Shah MF, Panteleimonitis S, Figueiredo N, Parvaiz A. Spotlight on laparoscopy in the surgical resection of locally advanced rectal cancer: multicenter propensity score match study. Ann Coloproctol. 2022; 38(4):307–313. DOI: 10.3393/ac.2020.01060.0151. PMID: 34399445. PMCID: PMC9441543.56. Piozzi GN, Kim SH. Robotic intersphincteric resection for low rectal cancer: technical controversies and a systematic review on the perioperative, oncological, and functional outcomes. Ann Coloproctol. 2021; 37(6):351–367. DOI: 10.3393/ac.2021.00836.0119. PMID: 34784706. PMCID: PMC8717069.57. Son GM, Ahn HM, Lee IY, Ha GW. Multifunctional indocyanine green applications for fluorescence-guided laparoscopic colorectal surgery. Ann Coloproctol. 2021; 37(3):133–140. DOI: 10.3393/ac.2021.05.07. PMID: 34102813. PMCID: PMC8273708.58. Hyun JH, Alhanafy MK, Park HC, Park SM, Park SC, Sohn DK, et al. Initial local excision for clinical T1 rectal cancer showed comparable overall survival despite high local recurrence rate: a propensity-matched analysis. Ann Coloproctol. 2022; 38(2):166–175. DOI: 10.3393/ac.2021.00479.0068. PMID: 34610653. PMCID: PMC9021851.59. van der Heijden JAG, van De Pas KGH, van den Broek FJC, Van Dielen FMH, Slooter GD, Maaskant-Braat AJG. Oncological and functional outcomes of transanal total mesorectal excision in a teaching hospital in the Netherlands. Ann Coloproctol. 2022; 38(1):28–35. DOI: 10.3393/ac.2020.00773.0110. PMID: 34182715. PMCID: PMC8898637.60. Kim S, Kang SI, Kim SH, Kim JH. The effect of anastomotic leakage on the incidence and severity of low anterior resection syndrome in patients undergoing proctectomy: a propensity score matching analysis. Ann Coloproctol. 2021; 37(5):281–290. DOI: 10.3393/ac.2021.03.15. PMID: 34098631. PMCID: PMC8566143.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects and Surgical Morbidity of Preoperative Combined Chemoradiotherapy for Locally Advanced Rectal Cancer
- Surgical issues in locally advanced rectal cancer treated by preoperative chemoradiotherapy
- An Update on Preoperative Radiotherapy for Locally Advanced Rectal Cancer
- Imaging Diagnosis of Locally Advanced Rectal Cancer: Tumor Staging before and after Preoperative Chemoradiotherapy
- Preoperative Concurrent Chemoradiotherapy with Oral Fluoropyrimidine in Locally Advanced Rectal Cancer: How Good Is Good Enough?