J Korean Med Sci.
2024 Jan;39(2):e5. 10.3346/jkms.2024.39.e5.
A Multicenter Analysis of Clinical Features and Long-Term Outcomes of POEMS Syndrome in Korea
- Affiliations
-
- 1Division of Hematology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Hematology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 6Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 7Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 8Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2550805
- DOI: http://doi.org/10.3346/jkms.2024.39.e5
Abstract
- Background
POEMS syndrome is a rare form of plasma cell dyscrasia characterized by polyneuropathy, organomegaly, endocrinopathy, monoclonal proteins, and skin changes. Owing to its low incidence, there are few reports regarding this syndrome. This multicenter study included 84 patients diagnosed with POEMS syndrome in South Korea.
Methods
We retrospectively evaluated 84 patients diagnosed with POEMS syndrome at 8 hospitals in South Korea between January 2000 and October 2022. The clinical characteristics and treatment outcomes were analyzed.
Results
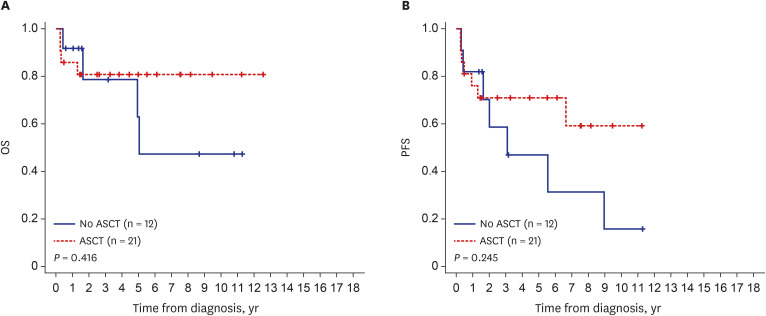
The median patient age was 53 years (range, 26–77 years), and 63.1% of the patients were male. All patients had peripheral neuropathy, and 81 (96.4%) had monoclonal plasma cell proliferation. Plasma vascular endothelial growth factor levels were available for 32 patients with a median of 821 pg/mL (range, 26–12,900 pg/mL). Other common features included skin changes (54.2%), volume overload (71.4%), and organomegaly (72.6%). Of the 84 patients, 75 received initial treatment (local radiotherapy, 6 [8.0%]; chemotherapy, 17 [22.7%]; both chemotherapy and local radiotherapy, 9 [12.0%]), upfront autologous stem cell transplantation (ASCT), 43 (57.3%; with induction chemotherapy, n = 12, 16.0%; without induction chemotherapy, n = 31, 41.3%). The median follow-up duration was 40.7 months. The 5-year overall survival (OS) was 78%, and the 5-year progression-free survival (PFS) was 55%. Patients who underwent upfront ASCT and were diagnosed after 2014 had a longer OS and PFS.
Conclusion
The demographics of Korean patients with POEMS syndrome were similar to those reported previously. Because of the introduction of new treatment agents and the reduced rate of transplant-related mortality related to ASCT, the treatment outcomes of Korean patients with POEMS syndrome have improved in recent years.
Figure
Reference
-
1. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996; 347(9002):702.2. Kanai K, Kuwabara S, Misawa S, Hattori T. Failure of treatment with anti-VEGF monoclonal antibody for long-standing POEMS syndrome. Intern Med. 2007; 46(6):311–313. PMID: 17380000.3. Samaras P, Bauer S, Stenner-Liewen F, Steiner R, Zweifel M, Renner C, et al. Treatment of POEMS syndrome with bevacizumab. Haematologica. 2007; 92(10):1438–1439. PMID: 18024383.4. D’Souza A, Hayman SR, Buadi F, Mauermann M, Lacy MQ, Gertz MA, et al. The utility of plasma vascular endothelial growth factor levels in the diagnosis and follow-up of patients with POEMS syndrome. Blood. 2011; 118(17):4663–4665. PMID: 21881050.5. Nakanishi T, Sobue I, Toyokura Y, Nishitani H, Kuroiwa Y, Satoyoshi E, et al. The Crow-Fukase syndrome: a study of 102 cases in Japan. Neurology. 1984; 34(6):712–720. PMID: 6539431.6. Kuwabara S, Dispenzieri A, Arimura K, Misawa S, Nakaseko C. Treatment for POEMS (polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes) syndrome. Cochrane Database Syst Rev. 2012; 2012(6):CD006828. PMID: 22696361.7. Jang IY, Yoon DH, Kim S, Lee K, Kim KK, Lim YM, et al. Advanced POEMS syndrome treated with high-dose melphalan followed by autologous blood stem cell transplantation: a single-center experience. Blood Res. 2014; 49(1):42–48. PMID: 24724066.8. Shim H, Seol CA, Park CJ, Cho YU, Seo EJ, Lee JH, et al. POEMS syndrome: bone marrow, laboratory, and clinical findings in 24 Korean patients. Ann Lab Med. 2019; 39(6):561–565. PMID: 31240884.9. Dispenzieri A. POEMS syndrome: 2021 update on diagnosis, risk-stratification, and management. Am J Hematol. 2021; 96(7):872–888. PMID: 34000085.10. Wang C, Huang XF, Cai QQ, Cao XX, Duan MH, Cai H, et al. Prognostic study for overall survival in patients with newly diagnosed POEMS syndrome. Leukemia. 2017; 31(1):100–106. PMID: 27338259.11. Kourelis TV, Buadi FK, Gertz MA, Lacy MQ, Kumar SK, Kapoor P, et al. Risk factors for and outcomes of patients with POEMS syndrome who experience progression after first-line treatment. Leukemia. 2016; 30(5):1079–1085. PMID: 26669974.12. D’Souza A, Lacy M, Gertz M, Kumar S, Buadi F, Hayman S, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012; 120(1):56–62. PMID: 22611150.13. Dispenzieri A. POEMS syndrome: 2017 update on diagnosis, risk stratification, and management. Am J Hematol. 2017; 92(8):814–829. PMID: 28699668.14. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15(12):e538–e548. PMID: 25439696.15. Suichi T, Misawa S, Beppu M, Takahashi S, Sekiguchi Y, Shibuya K, et al. Prevalence, clinical profiles, and prognosis of POEMS syndrome in Japanese nationwide survey. Neurology. 2019; 93(10):e975–e983. PMID: 31371568.16. Keddie S, Foldes D, Caimari F, Baldeweg SE, Bomsztyk J, Ziff OJ, et al. Clinical characteristics, risk factors, and outcomes of POEMS syndrome: a longitudinal cohort study. Neurology. 2020; 95(3):e268–e279. PMID: 32606227.17. Dispenzieri A, Kyle RA, Lacy MQ, Rajkumar SV, Therneau TM, Larson DR, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003; 101(7):2496–2506. PMID: 12456500.18. Kansagra A, Dispenzieri A, Fraser R, Estrada-Merly N, Sidana S, Nishihori T, et al. Outcomes after autologous hematopoietic cell transplantation in POEMS syndrome and comparison with multiple myeloma. Blood Adv. 2022; 6(13):3991–3995. PMID: 35507742.19. Li J, Duan MH, Wang C, Huang XF, Zhang W, Cao XX, et al. Impact of pretransplant induction therapy on autologous stem cell transplantation for patients with newly diagnosed POEMS syndrome. Leukemia. 2017; 31(6):1375–1381. PMID: 28100909.20. Cook G, Iacobelli S, van Biezen A, Ziagkos D, LeBlond V, Abraham J, et al. High-dose therapy and autologous stem cell transplantation in patients with POEMS syndrome: a retrospective study of the Plasma Cell Disorder sub-committee of the Chronic Malignancy Working Party of the European Society for Blood & Marrow Transplantation. Haematologica. 2017; 102(1):160–167. PMID: 27634201.21. Kawajiri-Manako C, Sakaida E, Ohwada C, Miyamoto T, Azuma T, Taguchi J, et al. Efficacy and long-term outcomes of autologous stem cell transplantation in POEMS syndrome: a nationwide survey in Japan. Biol Blood Marrow Transplant. 2018; 24(6):1180–1186. PMID: 29409882.22. Tomkins O, Keddie S, Lunn MP, D’Sa S. High-dose therapy and autologous transplantation for POEMS syndrome: effective, but how to optimise? Br J Haematol. 2019; 186(6):e178–e181. PMID: 31215033.23. Tokashiki T, Hashiguchi T, Arimura K, Eiraku N, Maruyama I, Osame M. Predictive value of serial platelet count and VEGF determination for the management of DIC in the Crow-Fukase (POEMS) syndrome. Intern Med. 2003; 42(12):1240–1243. PMID: 14714967.24. Kourelis TV, Dispenzieri A. Validation of a prognostic score for patients with POEMS syndrome: a mayo clinic cohort. Leukemia. 2017; 31(5):1251. PMID: 28280263.25. Kim YR. Update on the POEMS syndrome. Blood Res. 2022; 57:27–31. PMID: 35483922.26. Khwaja J, D’Sa S, Lunn MP, Sive J. Evidence-based medical treatment of POEMS syndrome. Br J Haematol. 2023; 200(2):128–136. PMID: 35934319.