Cancer Res Treat.
2024 Jan;56(1):134-142. 10.4143/crt.2023.652.
Toremifene, an Alternative Adjuvant Endocrine Therapy, Is Better Than Tamoxifen in Breast Cancer Patients with CYP2D6*10 Mutant Genotypes
- Affiliations
-
- 1Department of Breast Surgery, Harbin Medical University Cancer Hospital, Harbin, China
- 2Department of Clinical Medicine, Jiamusi University, Jiamusi, Heilongjiang, China
- 3Depatment of Thyroid and Breast Surgery, Ningbo Medical Center, Li Huili Hospital, Ningbo, China
- KMID: 2550330
- DOI: http://doi.org/10.4143/crt.2023.652
Abstract
- Purpose
Tamoxifen showed individual differences in efficacy under different CYP2D6*10 genotypes. Our study evaluated the prognosis of tamoxifen or toremifene in hormone receptor (HR)–positive breast cancer patients under different genotypes.
Materials and Methods
CYP2D6*10 genotypes of HR-positive breast cancer patients were determined by Sanger sequencing, and all the patients were divided into tamoxifen group or toremifene group.
Results
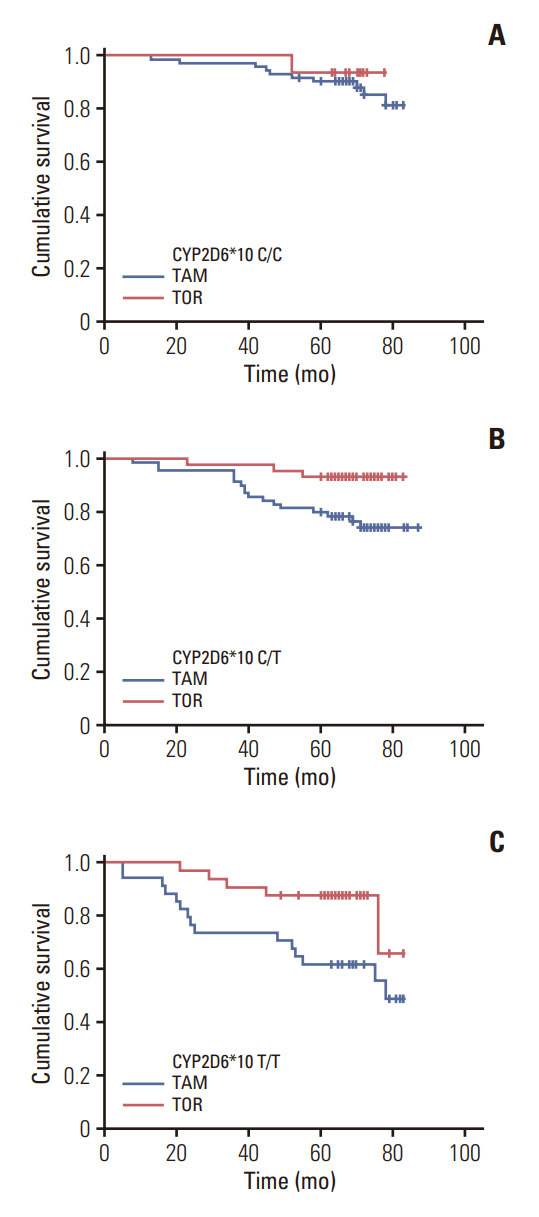
A total of 268 patients with HR-positive breast cancer were studied. The median follow-up time was 72.0 months (range, 5.0 to 88.0 months). Of these, 88 (32.9%), 114 (42.5%), and 66 (24.6%) patients had C/C, C/T, and T/T genotypes, respectively. Among patients who received tamoxifen (n=176), the 5-year disease-free survival (DFS) rate in patients with C/C and C/T genotype was better than that in patients with T/T genotype, and the difference was statistically significant (p < 0.001 and p=0.030, respectively). In patients receiving toremifene, CYP2D6*10 genotype was not significantly associated with DFS (p=0.325). Regardless of genotypes, the 5-year DFS rate was higher in patients treated with toremifene than in patients with tamoxifen (91.3% vs. 80.0%, p=0.011). Compared with tamoxifen, toremifene remained an independent prognostic marker of DFS in multivariate analysis (hazard ratio, 0.422; p=0.021). For all the 180 patients with CYP2D6*10 C/T and T/T genotypes, the 5-year DFS rate was significantly higher in the toremifene group than in the tamoxifen group (90.8% vs. 70.1%, p=0.003).
Conclusion
Toremifene may be an alternative adjuvant endocrine therapy for patients with CYP2D6*10 mutant genotypes.
Figure
Reference
-
References
1. O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010; 16:6100–10.2. Dezentje VO, Guchelaar HJ, Nortier JW, van de Velde CJ, Gelderblom H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin Cancer Res. 2009; 15:15–21.3. Jackisch C, Harbeck N, Huober J, von Minckwitz G, Gerber B, Kreipe HH, et al. 14th St. Gallen International Breast Cancer Conference 2015: evidence, controversies, consensus - primary therapy of early breast cancer: opinions expressed by German experts. Breast Care (Basel). 2015; 10:211–9.4. Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005; 97:1652–62.5. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–717.6. Blackburn HL, Ellsworth DL, Shriver CD, Ellsworth RE. Role of cytochrome P450 genes in breast cancer etiology and treatment: effects on estrogen biosynthesis, metabolism, and response to endocrine therapy. Cancer Causes Control. 2015; 26:319–32.7. Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014; 95:376–82.8. Qian JC, Xu XM, Hu GX, Dai DP, Xu RA, Hu LM, et al. Genetic variations of human CYP2D6 in the Chinese Han population. Pharmacogenomics. 2013; 14:1731–43.9. Goetz MP, Rae JM, Suman VJ, Safgren SL, Ames MM, Visscher DW, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005; 23:9312–8.10. Ye QL, Zhai ZM. Toremifene and tamoxifen have similar efficacy in the treatment of patients with breast cancer: a meta-analysis of randomized trials. Mol Biol Rep. 2014; 41:751–6.11. Mustonen MV, Pyrhonen S, Kellokumpu-Lehtinen PL. Toremifene in the treatment of breast cancer. World J Clin Oncol. 2014; 5:393–405.12. Kim J, Coss CC, Barrett CM, Mohler ML, Bohl CE, Li CM, et al. Role and pharmacologic significance of cytochrome P-450 2D6 in oxidative metabolism of toremifene and tamoxifen. Int J Cancer. 2013; 132:1475–85.13. Watanabe M, Watanabe N, Maruyama S, Kawashiro T. Comparative metabolic study between two selective estrogen receptor modulators, toremifene and tamoxifen, in human liver microsomes. Drug Metab Pharmacokinet. 2015; 30:325–33.14. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34.15. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights into breast cancer in the East vs. the West: a review. JAMA Oncol. 2019; 5:1489–96.16. Lan B, Ma F, Zhai X, Li Q, Chen S, Wang J, et al. The relationship between the CYP2D6 polymorphisms and tamoxifen efficacy in adjuvant endocrine therapy of breast cancer patients in Chinese Han population. Int J Cancer. 2018; 143:184–9.17. Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009; 302:1429–36.18. Lim HS, Lee HJ, Lee KS, Lee ES, Jang IJ, Ro J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J Clin Oncol. 2007; 25:3837–45.19. Helland T, Henne N, Bifulco E, Naume B, Borgen E, Kristensen VN, et al. Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res. 2017; 19:125.20. He W, Grassmann F, Eriksson M, Eliasson E, Margolin S, Thoren L, et al. CYP2D6 genotype predicts tamoxifen discontinuation and prognosis in patients with breast cancer. J Clin Oncol. 2020; 38:548–57.21. Sanchez-Spitman A, Dezentje V, Swen J, Moes D, Bohringer S, Batman E, et al. Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J Clin Oncol. 2019; 37:636–46.22. Goetz MP, Suman VJ, Nakamura Y, Kiyotani K, Jordan VC, Ingle JN. Tamoxifen metabolism and breast cancer recurrence: a question unanswered by CYPTAM. J Clin Oncol. 2019; 37:1982–3.23. Gusella M, Pasini F, Corso B, Bertolaso L, De Rosa G, Falci C, et al. Predicting steady-state endoxifen plasma concentrations in breast cancer patients by CYP2D6 genotyping or phenotyping: which approach is more reliable? Pharmacol Res Perspect. 2020; 8:e00646.24. Brauch H, Schroth W, Murdter T, Schwab M. Tamoxifen pharmacogenetics and metabolism: the same is not the same. J Clin Oncol. 2019; 37:1981–2.25. Jorge-Aaron RM, Rodrigo RC, de Jesus AI, Esther MR. CYP2D6 does not impact on breast cancer-free survival in Southeast Mexican patients under tamoxifen treatment. Per Med. 2020; 17:261–70.26. International Breast Cancer Study Group, Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, et al. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol. 2004; 15:1749–59.27. Holli K; Finnish Breast Cancer Group. Tamoxifen versus toremifene in the adjuvant treatment of breast cancer. Eur J Cancer. 2002; 38 Suppl 6:S37–8.28. Lewis JD, Chagpar AB, Shaughnessy EA, Nurko J, McMasters K, Edwards MJ. Excellent outcomes with adjuvant toremifene or tamoxifen in early stage breast cancer. Cancer. 2010; 116:2307–15.29. Zhou WB, Ding Q, Chen L, Liu XA, Wang S. Toremifene is an effective and safe alternative to tamoxifen in adjuvant endocrine therapy for breast cancer: results of four randomized trials. Breast Cancer Res Treat. 2011; 128:625–31.30. Ahn SH, Son BH, Kim SW, Kim SI, Jeong J, Ko SS, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea: a report from the Korean Breast Cancer Society. J Clin Oncol. 2007; 25:2360–8.31. Gu R, Jia W, Zeng Y, Rao N, Hu Y, Li S, et al. A comparison of survival outcomes and side effects of toremifene or tamoxifen therapy in premenopausal estrogen and progesterone receptor positive breast cancer patients: a retrospective cohort study. BMC Cancer. 2012; 12:161.32. Lan B, Ma F, Chen S, Wang W, Li Q, Fan Y, et al. Toremifene, rather than tamoxifen, might be a better option for the adjuvant endocrine therapy in CYP2D6*10T/T genotype breast cancer patients in China. Int J Cancer. 2018; 143:2499–504.33. Khalaj Z, Baratieh Z, Nikpour P, Schwab M, Schaeffeler E, Mokarian F, et al. Clinical trial: CYP2D6 related dose escalation of tamoxifen in breast cancer patients with Iranian ethnic background resulted in increased concentrations of tamoxifen and its metabolites. Front Pharmacol. 2019; 10:530.34. Tamura K, Imamura CK, Takano T, Saji S, Yamanaka T, Yonemori K, et al. CYP2D6 genotype-guided tamoxifen dosing in hormone receptor-positive metastatic breast cancer (TARGET-1): a randomized, open-label, phase II study. J Clin Oncol. 2020; 38:558–66.35. Goetz MP, Sangkuhl K, Guchelaar HJ, Schwab M, Province M, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin Pharmacol Ther. 2018; 103:770–7.36. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019; 30:1194–220.37. Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015; 372:436–46.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Toremifene as an Adjuvant Hormone Therapy for Estrogen Receptor Positive early Breast Cancer: Therapeutic Efficacy and Effect on Endometrium
- Comparison of Tamoxifen and Toremifene as Adjuvant Treatment in Node-negative Postmenopausal Breast Cancer
- Endometrial mullerian adenosarcoma after toremifene treatment in breast cancer patients: a case report
- Adjuvant Hormonal Therapy: Current Standard and Practical Issues
- Toremifene-associated endometrial polyp: A case report and review of the literature