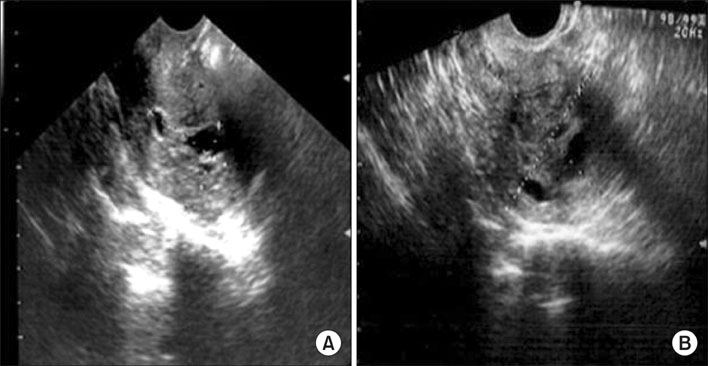
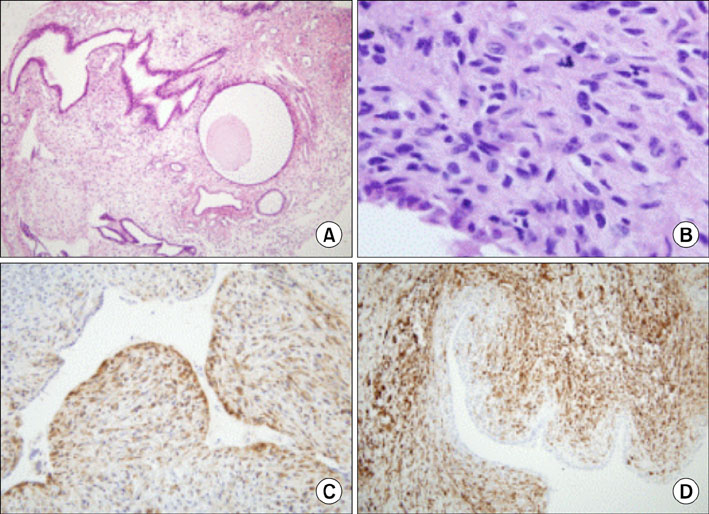
J Gynecol Oncol.
2010 Dec;21(4):269-272. 10.3802/jgo.2010.21.4.269.
Endometrial mullerian adenosarcoma after toremifene treatment in breast cancer patients: a case report
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea. kumcjskang@hanmail.net
- 2Department of Pathology, Korea University College of Medicine, Seoul, Korea.
- KMID: 2173552
- DOI: http://doi.org/10.3802/jgo.2010.21.4.269
Abstract
- Toremifene is an anti-estrogen which has been shown to be effective in the treatment of breast cancer, and is thought to be a less uterotrophic agent than tamoxifen. The risk assessment concerning endometrial cancer has been inconclusive because of its rare use up to the mid-1990s. We report a case of an adenosarcoma, which is a very rare type of uterine malignancy, after toremifene treatment for 5 years in a breast cancer patient. After 1 year of toremifene use, the patient had a benign Mullerian adenofibroma. After an additional 4 years of toremifene treatment, the endometrial polypoid lesion was transformed into a Mullerian adenosarcoma. Although toremifene is a promising anti-estrogenic agent in the treatment of breast cancer patients, clinicians should not neglect the possibility of a uterine malignancy.
MeSH Terms
Figure
Reference
-
1. Konar H, Biswas PK, Choudhury P, Roy M. Gynecological follow up of women with long term tamoxifen therapy for carcinoma breast. J Obstet Gynecol India. 2006. 56:333–336.2. Arenas M, Rovirosa A, Hernandez V, Ordi J, Jorcano S, Mellado B, et al. Uterine sarcomas in breast cancer patients treated with tamoxifen. Int J Gynecol Cancer. 2006. 16:861–865.3. Arici DS, Aker H, Yildiz E, Tasyurt A. Mullerian adenosarcoma of the uterus associated with tamoxifen therapy. Arch Gynecol Obstet. 2000. 264:105–107.4. Chourmouzi D, Boulogianni G, Zarampoukas T, Drevelengas A. Sonography and MRI of tamoxifen-associated mullerian adenosarcoma of the uterus. AJR Am J Roentgenol. 2003. 181:1673–1675.5. Carvalho FM, Carvalho JP, Motta EV, Souen J. Mullerian adenosarcoma of the uterus with sarcomatous overgrowth following tamoxifen treatment for breast cancer. Rev Hosp Clin Fac Med Sao Paulo. 2000. 55:17–20.6. Holli K, Valavaara R, Blanco G, Kataja V, Hietanen P, Flander M, et al. Safety and efficacy results of a randomized trial comparing adjuvant toremifene and tamoxifen in postmenopausal patients with node-positive breast cancer: Finnish Breast Cancer Group. J Clin Oncol. 2000. 18:3487–3494.7. Harvey HA, Kimura M, Hajba A. Toremifene: an evaluation of its safety profile. Breast. 2006. 15:142–157.8. Ha HK, Han W, Ko E, Kang SY, Lee JW, Cho J, et al. Toremifene as an adjuvant hormone therapy for estrogen receptor positive early breast cancer: therapeutic efficacy and effect on endometrium. J Breast Cancer. 2007. 10:258–262.9. Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, Simoncini E, et al. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12-93 and 14-93. Ann Oncol. 2004. 15:1749–1759.10. Stenbygaard LE, Herrstedt J, Thomsen JF, Svendsen KR, Engelholm SA, Dombernowsky P. Toremifene and tamoxifen in advanced breast cancer: a double-blind cross-over trial. Breast Cancer Res Treat. 1993. 25:57–63.11. Gianni L, Gelber S, Ravaioli A, Price KN, Panzini I, Fantini M, et al. Second non-breast primary cancer following adjuvant therapy for early breast cancer: a report from the International Breast Cancer Study Group. Eur J Cancer. 2009. 45:561–571.12. Marttunen MB, Cacciatore B, Hietanen P, Pyrhonen S, Tiitinen A, Wahlstrom T, et al. Prospective study on gynaecological effects of two antioestrogens tamoxifen and toremifene in postmenopausal women. Br J Cancer. 2001. 84:897–902.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MR Imaging Findings of Tamoxifen-associated Uterine Adenosarcoma: Report of Two Cases
- Toremifene-associated endometrial polyp: A case report and review of the literature
- A case of mullerian adenosarcoma of the uterus
- Toremifene as an Adjuvant Hormone Therapy for Estrogen Receptor Positive early Breast Cancer: Therapeutic Efficacy and Effect on Endometrium
- Mullerian Adenosarcoma Arising From Rectal Endometriosis