Ann Surg Treat Res.
2023 Dec;105(6):360-368. 10.4174/astr.2023.105.6.360.
A simplified risk scoring system for predicting high-risk groups in gene expression tests for patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative, and node-positive breast cancer
- Affiliations
-
- 1Department of Surgery, Gangneung Asan Medical Center, Gangneung, Korea
- 2Division of Breast Surgery, Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
- 3Yonsei University Graduate School of Medicine, Seoul, Korea
- KMID: 2548780
- DOI: http://doi.org/10.4174/astr.2023.105.6.360
Abstract
- Purpose
The gene expression test (GET) was used to predict the response to chemotherapy and the recurrence risk. Several randomized clinical trials have demonstrated that some patients with node-positive disease can achieve favorable survival outcomes even without adjuvant chemotherapy. This study aimed to predict the results of Oncotype DX (Genomic Health) and MammaPrint (Agendia) using traditional clinicopathological factors.
Methods
We reviewed the records of 311 patients who underwent GET for hormone receptor-positive/human epidermal growth factor receptor 2 (HER2)-negative primary invasive breast cancer with node-positive disease between 2015 and 2022 at Severance Hospital and Gangneung Asan Medical Center. Univariate and multivariate logistic regression analyses assessed the relationships between clinicopathological variables and risk stratification using the GET results.
Results
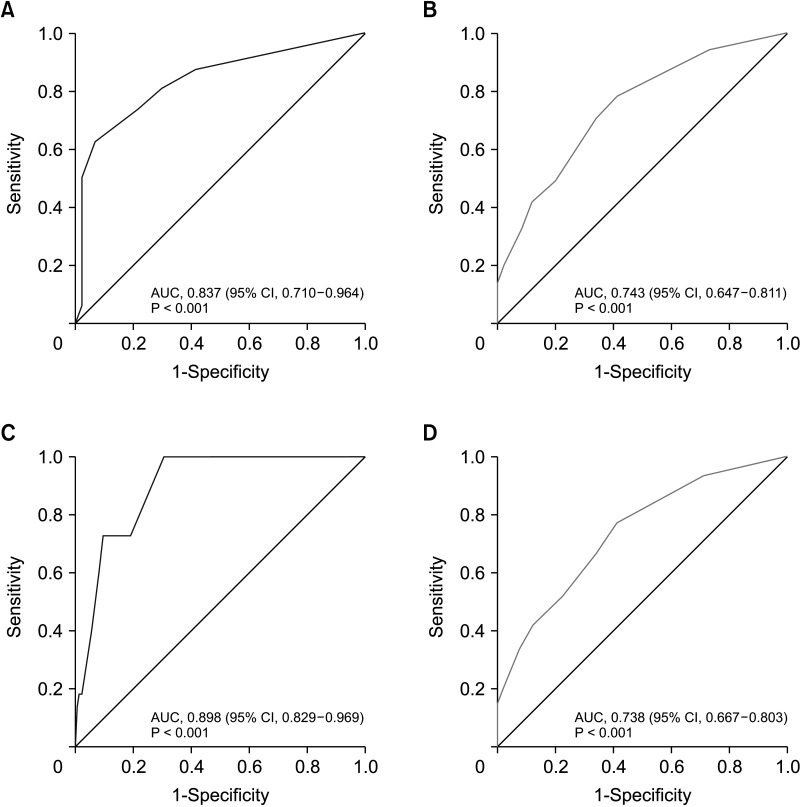
A simple scoring system was created by assigning integer values to each variable. A score of 3 was assigned for histological grade 3, a score of 2 for pathologic T2 or above, and a score of 1 for a lower progesterone receptor (1–20 or Alled score 3–6), HER2 2-positive, and high Ki-67 (>20). In the validation cohort, overall accuracy was 0.798 (95% confidence interval, 0.744–0.844).
Conclusion
The high GET risk results can be predicted using traditional clinicopathological factors: tumor size, progesterone receptor, histological grade, HER2, and Ki-67. These results will be useful for treatment decision-making among clinically high-risk patients with HR-positive/HER2-negative and node-positive disease, helping to identify patients to whom the GET assay may not apply.
Figure
Reference
-
1. Chen C, Dhanda R, Tseng WY, Forsyth M, Patt DA. Evaluating use characteristics for the oncotype dx 21-gene recurrence score and concordance with chemotherapy use in early-stage breast cancer. J Oncol Pract. 2013; 9:182–187. PMID: 23942918.
Article2. Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: an NSABP update. Cancer. 1983; 52:1551–1557. PMID: 6352003.
Article3. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991; 19:403–410. PMID: 1757079.
Article4. Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004; 96:218–228. PMID: 14759989.
Article5. Choi H, Ahn SG, Bae SJ, Kim JH, Eun NL, Lee Y, et al. Comparison of programmed cell death ligand 1 status between core needle biopsy and surgical specimens of triple-negative breast cancer. Yonsei Med J. 2023; 64:518–525. PMID: 37488704.
Article6. Lawn AM, Frampton AE, Krell J, Waheed S, Stacey-Clear A. Lymph node ratio can further stratify prognosis in subpopulations of breast cancer patients with axillary nodal metastases. Future Oncol. 2013; 9:1425–1431. PMID: 24106893.
Article7. Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016; 375:717–729. PMID: 27557300.
Article8. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021; 385:2336–2347. PMID: 34914339.
Article9. Caparica R, Brandão M, Piccart M. Systemic treatment of patients with early breast cancer: recent updates and state of the art. Breast. 2019; 48 Suppl 1:S7–S20. PMID: 31839166.
Article10. National Comprehensive Cancer Network (NCCN). NCCN Guidelines version 4.2023: Breast Cancer [Internet]. NCCN;2023. cited 2023 Jul 1. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.11. Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010; 28:1829–1834. PMID: 20212256.
Article12. Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006; 8:R25. PMID: 16737553.
Article13. Andre F, Ismaila N, Allison KH, Barlow WE, Collyar DE, Damodaran S, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol. 2022; 40:1816–1837. PMID: 35439025.
Article14. Lim H, Kim SI, Hyun S, Lee GB, Seol A, Lee M. Uptake rate of risk-reducing salpingo-oophorectomy and surgical outcomes of female germline BRCA1/2 mutation carriers: a retrospective cohort study. Yonsei Med J. 2021; 62:1090–1097. PMID: 34816639.
Article15. Eaton AA, Pesce CE, Murphy JO, Stempel MM, Patil SM, Brogi E, et al. Estimating the OncotypeDX score: validation of an inexpensive estimation tool. Breast Cancer Res Treat. 2017; 161:435–441. PMID: 27928699.
Article16. Lee SB, Kim J, Sohn G, Kim J, Chung IY, Kim HJ, et al. A nomogram for predicting the oncotype DX recurrence score in women with T1-3N0-1miM0 hormone receptor positive, human epidermal growth factor 2 (HER2) negative breast cancer. Cancer Res Treat. 2019; 51:1073–1085. PMID: 30384581.
Article17. Lee YJ, Hwang YS, Kim J, Ahn SH, Son BH, Kim HJ, et al. A nomogram for predicting probability of low risk of MammaPrint results in women with clinically high-risk breast cancer. Sci Rep. 2021; 11:23509. PMID: 34873249.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Value of Epidermal Growth Factor Receptor in Primary Breast Cancer
- MicroRNA-222 Expression as a Predictive Marker for Tumor Progression in Hormone Receptor-Positive Breast Cancer
- Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy
- Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- Subtype Is a Predictive Factor of Nonsentinel Lymph Node Involvement in Sentinel Node-Positive Breast Cancer Patients