J Breast Cancer.
2019 Jun;22(2):336-340. 10.4048/jbc.2019.22.e17.
Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University College of Medicine, Seoul, Korea. sooyoun.cho@samsung.com
- KMID: 2450126
- DOI: http://doi.org/10.4048/jbc.2019.22.e17
Abstract
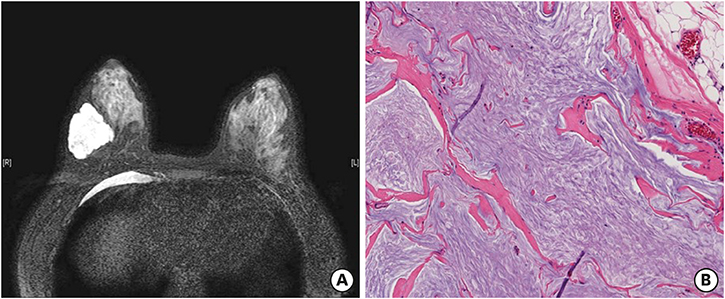
- Mucinous carcinoma (MC) is a rare subtype of breast cancer, which is composed of tumor cells floating in the abundant extracellular mucin. This form of cancer is usually estrogen receptor/progesterone receptor positive and human epidermal growth factor receptor 2 (HER2) negative. Here, we present a case of HER2-positive MC with an unusual signet ring cell differentiation. It is very rare that a breast tumor consists entirely of signet ring cells. The tumor showed pathologic complete response (pCR) after neoadjuvant chemotherapy with trastuzumab and pertuzumab. pCR of HER2-positive MC has rarely been described in literature. It is important to consider the biological heterogeneity of MCs for effective management.
Keyword
MeSH Terms
-
Adenocarcinoma, Mucinous
Breast Neoplasms
Carcinoma, Signet Ring Cell
Cell Differentiation*
Drug Therapy*
Epidermal Growth Factor*
Estrogens
Humans*
Mucins
Neoadjuvant Therapy
Polymerase Chain Reaction
Population Characteristics
Receptor, Epidermal Growth Factor*
Receptor, ErbB-2
Trastuzumab
Epidermal Growth Factor
Estrogens
Mucins
Receptor, Epidermal Growth Factor
Receptor, ErbB-2
Trastuzumab
Figure
Cited by 1 articles
-
Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
J Pathol Transl Med. 2020;54(1):95-102. doi: 10.4132/jptm.2019.10.24.
Reference
-
1. Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast. 4th ed. Lyon: International Agency for Research on Cancer;2012.2. Loibl S, Gianni L. HER2-positive breast cancer. Lancet. 2017; 389:2415–2429.
Article3. Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011; 14:308–313.
Article4. Pan B, Yao R, Shi J, Xu QQ, Zhou YD, Mao F, et al. Prognosis of subtypes of the mucinous breast carcinoma in Chinese women: a population-based study of 32-year experience (1983–2014). Oncotarget. 2016; 7:38864–38875.
Article5. Ranade A, Batra R, Sandhu G, Chitale RA, Balderacchi J. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010; 63:1043–1047.
Article6. Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010; 222:282–298.
Article7. Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical nomogram for predicting survival outcomes in early mucinous breast cancer. PLoS One. 2016; 11:e0164921.
Article8. Avisar E, Khan MA, Axelrod D, Oza K. Pure mucinous carcinoma of the breast: a clinicopathologic correlation study. Ann Surg Oncol. 1998; 5:447–451.
Article9. Ross JS, Gay LM, Nozad S, Wang K, Ali SM, Boguniewicz A, et al. Clinically advanced and metastatic pure mucinous carcinoma of the breast: a comprehensive genomic profiling study. Breast Cancer Res Treat. 2016; 155:405–413.
Article10. Bartosch C, Mendes N, Rios E, Rodrigues M, Eloy C, Reis CA, et al. Morphological features and mucin expression profile of breast carcinomas with signet-ring cell differentiation. Pathol Res Pract. 2015; 211:588–595.
Article11. Hull MT, Seo IS, Battersby JS, Csicsko JF. Signet-ring cell carcinoma of the breast: a clinicopathologic study of 24 cases. Am J Clin Pathol. 1980; 73:31–35.
Article12. Kim HM, Kim EK, Koo JS. Mucinous carcinoma with extensive signet ring cell differentiation: a case report. J Pathol Transl Med. 2017; 51:176–179.
Article13. Leung KM, Yeoh GP, Chan JK, Cheung PS, Chan KW. Ductal type signet ring cell carcinoma of breast with growth pattern of pure mucinous carcinoma. Pathology. 2011; 43:282–284.
Article14. Baretta Z, Guindalini RS, Khramtsova G, Olopade OI. Resistance to trastuzumab in HER2-positive mucinous invasive ductal breast carcinoma. Clin Breast Cancer. 2013; 13:156–158.
Article15. Didonato R, Shapiro N, Koenigsberg T, D'Alfonso T, Jaffer S, Fineberg S. Invasive mucinous carcinoma of the breast and response patterns after neoadjuvant chemotherapy (NAC). Histopathology. 2018; 72:965–973.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mucinous Carcinoma with Extensive Signet Ring Cell Differentiation: A Case Report
- Cytologic Features of Signet Ring Cell Carcinoma of the Uterine Cervix: A Report of Two Cases
- Two Cases of Invasive Carcinoma of the Breast Composed Mostly of Signet Ring Cells in the Fine Needle Aspiration Cytology
- Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- Prognostic significance of epidermal growth factor receptor expression in human gastric carcinoma