Neonatal Med.
2023 Nov;30(4):96-101. 10.5385/nm.2023.30.4.96.
Does Neonatal Microbiome Research Encompass the Placental Transfer Pathway?
- Affiliations
-
- 1Department of Pediatrics, Kyung Hee University Hospital, College of Medicine, Kyung Hee University, Seoul, Korea
- KMID: 2548299
- DOI: http://doi.org/10.5385/nm.2023.30.4.96
Abstract
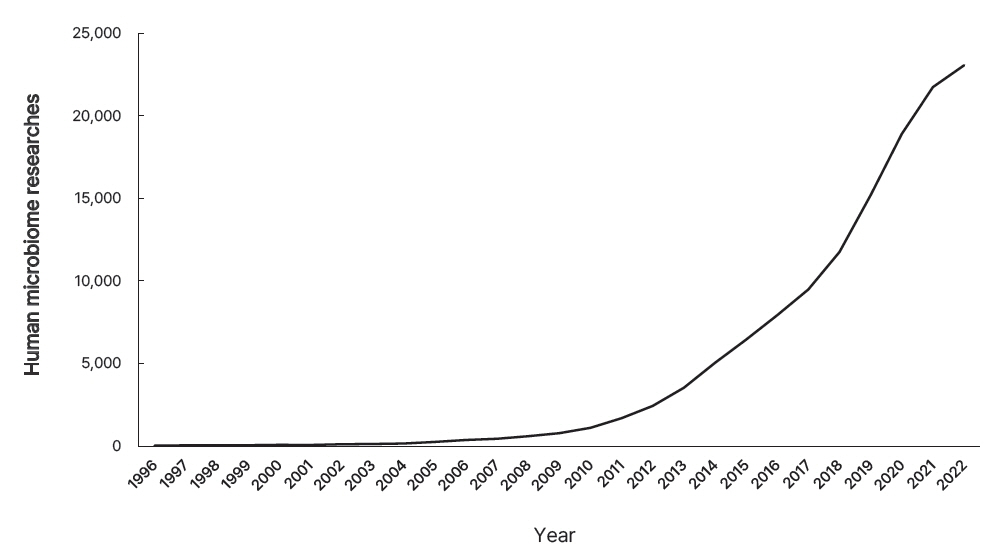
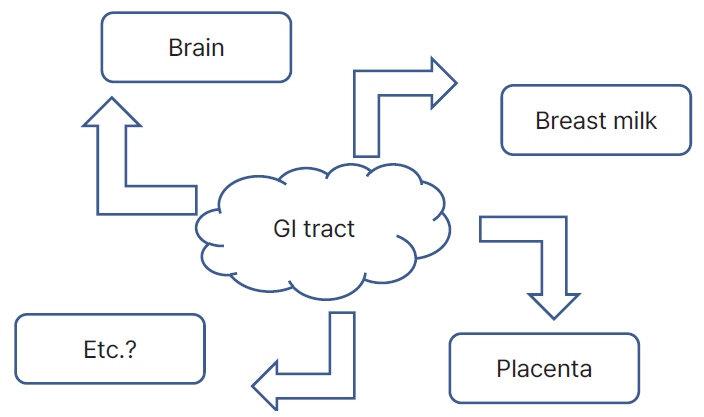
- The word “microbiome” is a combination of “microbiota” and “genome,” which represents the genomic concept of microbiota. The bacterial culture method is the mainstay of identifying microbes, while polymerase chain reaction adds diagnostic value. However, in the era of next-generation sequencing, achieving high-throughput microbiota footprints is extremely sensitive. This sensitivity often leads to confusion, as it can detect specific microbes genomes, even in sterile samples, such as blood, placenta, breast milk, skin, vagina, and stool. The neonatal microbiome remarkably influences both fetal and neonatal life related to health status and disease outcome. However, its origins pose a question: does it stem from a direct gateway or through a breakdown of barriers? This review provides a brief overview of evidence and speculative insights.
Keyword
Figure
Reference
-
1. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012; 486:207–14.2. Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016; 8:51.
Article3. Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004; 53:1617–23.
Article4. Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009; 7:1202–9.
Article5. Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010; 105:2218–27.
Article6. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368:407–15.
Article7. Rossen NG, Fuentes S, van der Spek MJ, Tijssen JG, Hartman JH, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149:110–8.
Article8. Davenport ER, Sanders JG, Song SJ, Amato KR, Clark AG, Knight R. The human microbiome in evolution. BMC Biol. 2017; 15:127.
Article9. Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol. 2016; 16:86.
Article10. Feldman-Winter L, Barone L, Milcarek B, Hunter K, Meek J, Morton J, et al. Residency curriculum improves breastfeeding care. Pediatrics. 2010; 126:289–97.
Article11. Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999; 28:19–25.
Article12. Huurre A, Kalliomaki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery: effects on gut microbiota and humoral immunity. Neonatology. 2008; 93:236–40.
Article13. Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014; 63:559–66.
Article14. Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017; 171:647–54.
Article15. Kim H, Sitarik AR, Woodcroft K, Johnson CC, Zoratti E. Birth mode, breastfeeding, pet exposure, and antibiotic use: associations with the gut microbiome and sensitization in children. Curr Allergy Asthma Rep. 2019; 19:22.
Article16. Gregory KE, Samuel BS, Houghteling P, Shan G, Ausubel FM, Sadreyev RI, et al. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016; 4:68.
Article17. Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018; 562:583–8.
Article18. Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017; 5:48.
Article19. Stinson LF, Payne MS, Keelan JA. Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. 2017; 43:352–69.
Article20. La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A. 2014; 111:12522–7.
Article21. Moles L, Gomez M, Heilig H, Bustos G, Fuentes S, de Vos W, et al. Bacterial diversity in meconium of preterm neonates and evolution of their fecal microbiota during the first month of life. PLoS One. 2013; 8:e66986.
Article22. Morais J, Marques C, Teixeira D, Durao C, Faria A, Brito S, et al. Extremely preterm neonates have more Lactobacillus in meconium than very preterm neonates: the in utero microbial colonization hypothesis. Gut Microbes. 2020; 12:1785804.23. Zakis DR, Paulissen E, Kornete L, Kaan AM, Nicu EA, Zaura E. The evidence for placental microbiome and its composition in healthy pregnancies: a systematic review. J Reprod Immunol. 2022; 149:103455.
Article24. Lim ES, Rodriguez C, Holtz LR. Amniotic fluid from healthy term pregnancies does not harbor a detectable microbial community. Microbiome. 2018; 6:87.
Article25. Glassing A, Dowd SE, Galandiuk S, Davis B, Chiodini RJ. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog. 2016; 8:24.
Article26. Olomu IN, Pena-Cortes LC, Long RA, Vyas A, Krichevskiy O, Luellwitz R, et al. Elimination of “kitome” and “splashome” contamination results in lack of detection of a unique placental microbiome. BMC Microbiol. 2020; 20:157.
Article27. Williams N, Vella R, Zhou Y, Gao H, Mass K, Townsel C, et al. Investigating the origin of the fetal gut and placenta microbiome in twins. J Matern Fetal Neonatal Med. 2022; 35:7025–35.
Article28. Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014; 6:237ra65.
Article29. Khodayar-Pardo P, Mira-Pascual L, Collado MC, Martinez-Costa C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J Perinatol. 2014; 34:599–605.
Article30. Yi DY, Kim SY. Human breast milk composition and function in human health: from nutritional components to microbiome and microRNAs. Nutrients. 2021; 13:3094.
Article31. Ramsay DT, Kent JC, Owens RA, Hartmann PE. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics. 2004; 113:361–7.
Article32. Williams JE, Carrothers JM, Lackey KA, Beatty NF, Brooker SL, Peterson HK, et al. Strong multivariate relations exist among milk, oral, and fecal microbiomes in mother-infant dyads during the first six months postpartum. J Nutr. 2019; 149:902–14.
Article33. Hassiotou F, Hepworth AR, Metzger P, Tat Lai C, Trengove N, Hartmann PE, et al. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin Transl Immunology. 2013; 2:e3.
Article34. Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol. 2014; 16:2891–904.
Article35. Lackey KA, Williams JE, Meehan CL, Zachek JA, Benda ED, Price WJ, et al. What’s normal?: microbiomes in human milk and infant feces are related to each other but vary geographically: the INSPIRE Study. Front Nutr. 2019; 6:45.
Article36. Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. 2023; 7:CD005496.
Article37. Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005; 115:696–703.
Article38. Lu J, Lu L, Yu Y, Cluette-Brown J, Martin CR, Claud EC. Effects of intestinal microbiota on brain development in humanized gnotobiotic mice. Sci Rep. 2018; 8:5443.
Article39. Lu J, Lu L, Yu Y, Oliphant K, Drobyshevsky A, Claud EC. Early preterm infant microbiome impacts adult learning. Sci Rep. 2022; 12:3310.
Article40. Ahmed H, Leyrolle Q, Koistinen V, Karkkainen O, Laye S, Delzenne N, et al. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes. 2022; 14:2102878.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Applications of the Microbiome in Obstetrics
- Endogenous Digoxin-like Substance in Neonatal Serum, Pregnant Women and Placental Extracts
- Fetal and preterm infant microbiomes: a new perspective of necrotizing enterocolitis
- Clinical Significance of Large Placental Chorioangioma
- The Relationship between Placental Ratio and Neonatal Morbidity in Intrauterine Growth Restricted Infants