Restor Dent Endod.
2021 Aug;46(3):e35. 10.5395/rde.2021.46.e35.
Comparison of the sealing ability of various bioceramic materials for endodontic surgery
- Affiliations
-
- 1Department of Endodontics, School of Dentistry, University of Washington, Seattle, WA, USA
- 2Department of Periodontics, University of Washington, Seattle, WA, USA
- 3Department of Material Science and Engineering, University of Washington, Seattle, WA, USA
- KMID: 2548079
- DOI: http://doi.org/10.5395/rde.2021.46.e35
Abstract
Objectives
Endosequence Bioceramic Root Repair Material (BC-RRM) is used in endodontic microsurgery. It is available as a paste and a putty. However, no studies to date have examined the sealing ability of these forms alone or in combination as root-end filling materials. Hence, this study aimed to compare the sealing properties of these 2 forms of BC-RRM.
Materials and Methods
Forty-two extracted upper anterior teeth were divided into 3 experimental groups, a positive and negative control. After the root canal treatment, the root ends were resected, retroprepared and retrofilled with either putty, paste + putty or mineral trioxide aggregate (MTA). The teeth were mounted in tubes so the apical 3 mm was submerged in Brain Heart Infusion (BHI) broth. The coronal portions of the canals were inoculated with Enterococcus faecalis and BHI broth and incubated for 30 days. The broth in the tubes was analyzed for colony forming units to check for leakage of bacteria from the canal. The teeth from the groups were sectioned and analyzed using scanning electron microscopy (SEM). The Kruskal-Wallis test and analysis of variance were used to analyze the data with a significance level p < 0.05.
Results
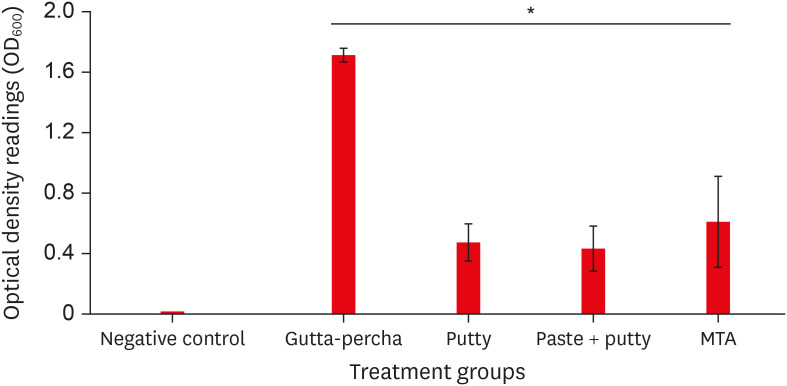
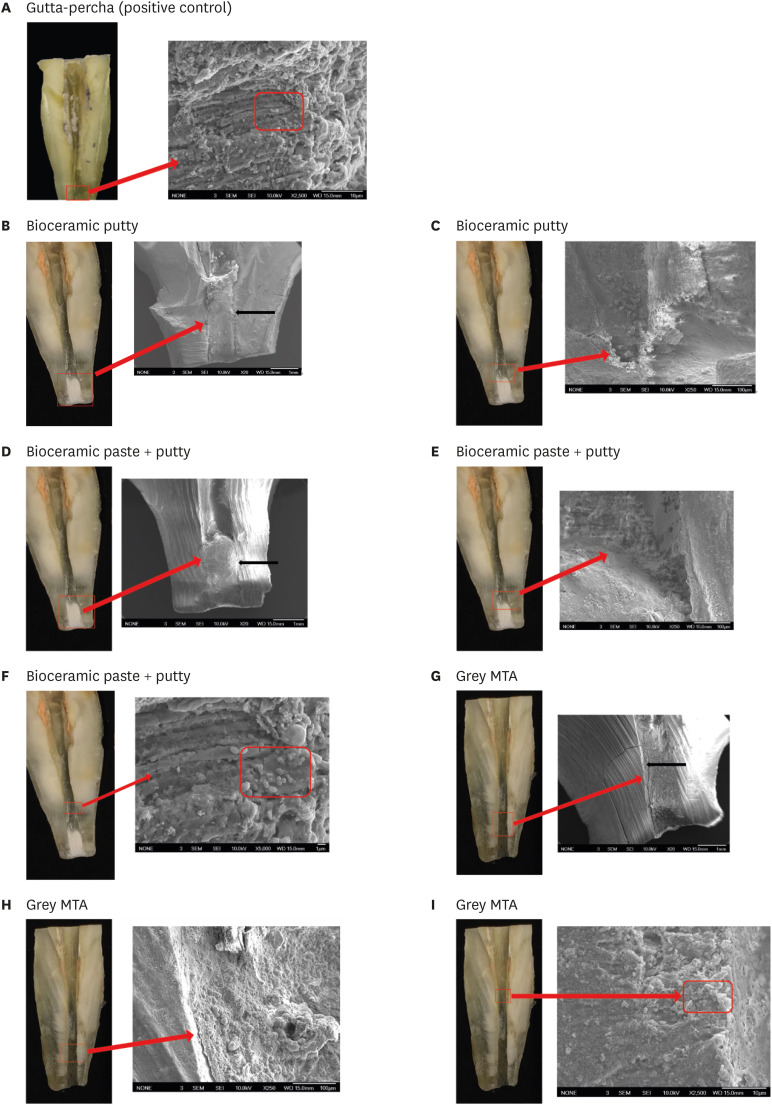
The BC-RRM and MTA groups showed similar sealing ability. The positive control showed leakage in all samples. The SEM imaging showed the presence of bacteria in all experimental groups at the material-tooth interface.
Conclusions
No significant differences were noted in the experimental groups, providing sufficient evidence that any combination could be effectively used during endodontic microsurgery.
Figure
Reference
-
1. Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006; 32:601–623. PMID: 16793466.
Article2. Huang S, Chen NN, Yu VSH, Lim HA, Lui JN. Long-term success and survival of endodontic microsurgery. J Endod. 2020; 46:149–157.e4. PMID: 31879031.
Article3. Safi C, Kohli MR, Kratchman SI, Setzer FC, Karabucak B. Outcome of endodontic microsurgery using mineral trioxide aggregate or root repair material as root-end filling material: a randomized controlled trial with cone-beam computed tomographic evaluation. J Endod. 2019; 45:831–839. PMID: 31078325.
Article4. Siqueira JF Jr, Rôças IN, Ricucci D, Hülsmann M. Causes and management of post-treatment apical periodontitis. Br Dent J. 2014; 216:305–312. PMID: 24651336.
Article5. Torabinejad M, Pitt Ford TR. Root end filling materials: a review. Endod Dent Traumatol. 1996; 12:161–178. PMID: 9028180.
Article6. Chong BS, Pitt Ford TR, Watson TF, Wilson RF. Sealing ability of potential retrograde root filling materials. Endod Dent Traumatol. 1995; 11:264–269. PMID: 8617160.
Article7. Saxena P, Gupta SK, Newaskar V. Biocompatibility of root-end filling materials: recent update. Restor Dent Endod. 2013; 38:119–127. PMID: 24010077.
Article8. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. J Endod. 2010; 36:190–202. PMID: 20113774.
Article9. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413. PMID: 20171353.
Article10. Butt N, Talwar S, Chaudhry S, Nawal RR, Yadav S, Bali A. Comparison of physical and mechanical properties of mineral trioxide aggregate and Biodentine. Indian J Dent Res. 2014; 25:692–697. PMID: 25728098.
Article11. Chen I, Karabucak B, Wang C, Wang HG, Koyama E, Kohli MR, Nah HD, Kim S. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod. 2015; 41:389–399. PMID: 25596728.
Article12. Abusrewil SM, McLean W, Scott JA. The use of Bioceramics as root-end filling materials in periradicular surgery: a literature review. Saudi Dent J. 2018; 30:273–282. PMID: 30202163.
Article13. Mahgoub N, Alqadasi B, Aldhorae K, Assiry A, Altawili ZM, Tao Hong . Comparison between iroot bp plus (endosequence root repair material) and mineral trioxide aggregate as pulp-capping agents: a systematic review. J Int Soc Prev Community Dent. 2019; 9:542–552. PMID: 32039073.
Article14. Lovato KF, Sedgley CM. Antibacterial activity of endosequence root repair material and proroot MTA against clinical isolates of Enterococcus faecalis . J Endod. 2011; 37:1542–1546. PMID: 22000459.
Article15. Alanezi AZ, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:e122–e125. PMID: 20219576.
Article16. Shinbori N, Grama AM, Patel Y, Woodmansey K, He J. Clinical outcome of endodontic microsurgery that uses EndoSequence BC root repair material as the root-end filling material. J Endod. 2015; 41:607–612. PMID: 25702859.
Article17. Allan NA, Walton RC, Schaeffer MA. Setting times for endodontic sealers under clinical usage and in vitro conditions. J Endod. 2001; 27:421–423. PMID: 11487139.
Article18. Gilheany PA, Figdor D, Tyas MJ. Apical dentin permeability and microleakage associated with root end resection and retrograde filling. J Endod. 1994; 20:22–26. PMID: 8182382.
Article19. Antunes HS, Gominho LF, Andrade-Junior CV, Dessaune-Neto N, Alves FR, Rôças IN, Siqueira JF Jr. Sealing ability of two root-end filling materials in a bacterial nutrient leakage model. Int Endod J. 2016; 49:960–965. PMID: 26334201.
Article20. Toia CC, Teixeira FB, Cucco C, Valera MC, Cavalcanti BN. Filling ability of three bioceramic root-end filling materials: a micro-computed tomography analysis. Aust Endod J. 2020; 46:424–431. PMID: 32895998.
Article21. Tran D, He J, Glickman GN, Woodmansey KF. Comparative analysis of calcium silicate-based root filling materials using an open apex model. J Endod. 2016; 42:654–658. PMID: 26925519.
Article22. Zamparini F, Siboni F, Prati C, Taddei P, Gandolfi MG. Properties of calcium silicate-monobasic calcium phosphate materials for endodontics containing tantalum pentoxide and zirconium oxide. Clin Oral Investig. 2019; 23:445–457.
Article23. Chong BS, Pitt Ford TR, Hudson MB. A prospective clinical study of mineral trioxide aggregate and IRM when used as root-end filling materials in endodontic surgery. Int Endod J. 2003; 36:520–526. PMID: 12887380.
Article24. Christiansen R, Kirkevang LL, Hørsted-Bindslev P, Wenzel A. Randomized clinical trial of root-end resection followed by root-end filling with mineral trioxide aggregate or smoothing of the orthograde gutta-percha root filling--1-year follow-up. Int Endod J. 2009; 42:105–114. PMID: 19134038.
Article25. Kruse C, Spin-Neto R, Christiansen R, Wenzel A, Kirkevang LL. Periapical bone healing after apicectomy with and without retrograde root filling with mineral trioxide aggregate: a 6-year follow-up of a randomized controlled trial. J Endod. 2016; 42:533–537. PMID: 26898567.
Article26. Lindeboom JA, Frenken JW, Kroon FH, van den Akker HP. A comparative prospective randomized clinical study of MTA and IRM as root-end filling materials in single-rooted teeth in endodontic surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100:495–500. PMID: 16200680.
Article27. Nair U, Ghattas S, Saber M, Natera M, Walker C, Pileggi R. A comparative evaluation of the sealing ability of 2 root-end filling materials: an in vitro leakage study using Enterococcus faecalis . Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:e74–e77. PMID: 21507688.28. Zhang C, Du J, Peng Z. Correlation between Enterococcus faecalis and persistent intraradicular infection compared with primary intraradicular infection: a systematic review. J Endod. 2015; 41:1207–1213. PMID: 26015157.
Article29. Song M, Jung IY, Lee SJ, Lee CY, Kim E. Prognostic factors for clinical outcomes in endodontic microsurgery: a retrospective study. J Endod. 2011; 37:927–933. PMID: 21689546.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A micro-computed tomographic study using a novel test model to assess the filling ability and volumetric changes of bioceramic root repair materials
- Perspective of endodontic sealers based on calcium silicate
- Bacterial leakage and micro-computed tomography evaluation in round-shaped canals obturated with bioceramic cone and sealer using matched single cone technique
- Retrograde filling with Lid technique in periapical surgery: case report
- An in Vitro Study of the Effects of Different Dentin Bonding Agents on the Prevention of Tooth Discoloration and the Sealing Ability of Calcium Silicate-Based Cement in Regenerative Endodontic Procedures