Clin Endosc.
2023 Nov;56(6):744-753. 10.5946/ce.2023.005.
Prevalence, natural progression, and clinical practices of upper gastrointestinal subepithelial lesions in Korea: a multicenter study
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Internal Medicine, Pusan National University School of Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 3Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- 4Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea
- 5Department of Internal Medicine, Inje University Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 6Department of Internal Medicine, Jeju National University College of Medicine, Jeju, Korea
- 7Department of Internal Medicine, Wonkwang University College of Medicine, Iksan, Korea
- 8Department of Internal Medicine, Research Institute of Clinical Medicine of Jeonbuk National University-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Korea
- 9Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 10EUS Study Group, Korean Society of Gastrointestinal Endoscopy, Seoul, Korea
- KMID: 2547896
- DOI: http://doi.org/10.5946/ce.2023.005
Abstract
- Background/Aims
This study aimed to evaluate the prevalence and natural progression of subepithelial lesions (SELs) in the upper gastrointestinal (UGI) tract.
Methods
The medical records of patients with UGI SELs who underwent endoscopic screening at eight university hospitals between January and December 2010 were retrospectively investigated. The follow-up evaluations were performed until December 2016.
Results
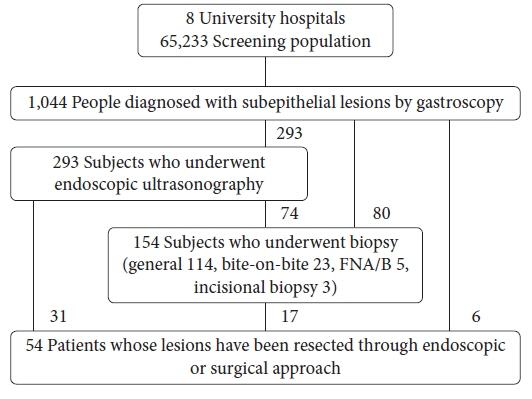
UGI SELs were found in 1,044 of the 65,233 participants screened (endoscopic prevalence, 1.60%; the total number of lesions, 1,062; mean age, 55.1±11.2 years; men, 53.6%). The median follow-up period was 48 (range, 8–74) months. SELs were most frequently found in the stomach (63.8%) and had a mean size of 9.9±6.1 mm. Endoscopic ultrasonography (EUS) was performed in 293 patients (28.1%). The most common lesions were leiomyomas, followed by gastrointestinal stromal tumors (GISTs), and ectopic pancreas. The proportions of SELs with malignant potential according to size were 3% (<1 cm), 22% (1–2 cm), 27% (2–3 cm), and 38% (≥3 cm). In gastric SELs larger than 1 cm, resections were performed in 20 patients because of an increase in size, of which 12 were found to be GISTs.
Conclusions
The prevalence of UGI SELs was 1.60%. Further, 23% of gastric SELs ≥1 cm were precancerous lesions, most followed by EUS and clinical decisions without initial pathological confirmation.
Figure
Cited by 1 articles
-
Endoscopically resected duodenal lipoma as an uncommon cause of upper gastrointestinal bleeding: a case report
Dong Chan Joo, Gwang Ha Kim, Bong Eun Lee, Moon Won Lee, Cheolung Kim
Ewha Med J. 2024;47(1):e8. doi: 10.12771/emj.2024.e8.
Reference
-
1. Lee M, Min BH, Lee H, et al. Feasibility and diagnostic yield of endoscopic ultrasonography-guided fine needle biopsy with a new core biopsy needle device in patients with gastric subepithelial tumors. Medicine (Baltimore). 2015; 94:e1622.
Article2. Kim GH, Cho YK, Kim EY, et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014; 49:347–354.
Article3. He G, Wang J, Chen B, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc. 2016; 30:4206–4213.
Article4. Standards of Practice Committee, Faulx AL, Kothari S, et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017; 85:1117–1132.
Article5. Gao Z, Wang C, Xue Q, et al. The cut-off value of tumor size and appropriate timing of follow-up for management of minimal EUS-suspected gastric gastrointestinal stromal tumors. BMC Gastroenterol. 2017; 17:8.
Article6. Ryu DG, Choi CW. Common gastric subepithelial tumors in Koreans. Korean J Helicobacter Up Gastrointest Res. 2022; 22:29–37.
Article7. Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010; 16:439–444.
Article8. Lee JH, Lee HL, Ahn YW, et al. Prevalence of gastric subepithelial tumors in Korea: a single center experience. Korean J Gastroenterol. 2015; 66:274–276.
Article9. Sharzehi K, Sethi A, Savides T. AGA clinical practice update on management of subepithelial lesions encountered during routine endoscopy: expert review. Clin Gastroenterol Hepatol. 2022; 20:2435–2443.
Article10. Moon JS. Role of endoscopic ultrasonography in guiding treatment plans for upper gastrointestinal subepithelial tumors. Clin Endosc. 2016; 49:220–225.
Article11. Kim SM, Kim EY, Cho JW, et al. Predictive factors for differentiating gastrointestinal stromal tumors from leiomyomas based on endoscopic ultrasonography findings in patients with gastric subepithelial tumors: a multicenter retrospective study. Clin Endosc. 2021; 54:872–880.
Article12. Bang CS, Baik GH, Shin IS, et al. Endoscopic submucosal dissection of gastric subepithelial tumors: a systematic review and meta-analysis. Korean J Intern Med. 2016; 31:860–871.
Article13. Kim TW, Kim GH, Park DY, et al. Endoscopic resection for duodenal subepithelial tumors: a single-center experience. Surg Endosc. 2017; 31:1936–1946.
Article14. Deprez PH, Moons LM, OʼToole D, et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022; 54:412–429.
Article15. Kim MN, Kang SJ, Kim SG, et al. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography. Gut Liver. 2013; 7:642–647.
Article16. Kim SE, Park MI. Natural course of gastric subepithelial tumor. Korean J Helicobacter Up Gastrointest Res. 2015; 15:1–8.
Article17. Cho JW; Korean ESD Study Group. Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin Endosc. 2016; 49:235–240.
Article18. Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc. 2016; 30:2431–2441.
Article19. Pouw RE, Barret M, Biermann K, et al. Endoscopic tissue sampling: Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021; 53:1174–1188.
Article20. Lee JS, Cho CM, Kwon YH, Seo AN, Bae HI, Han MH. Comparison of diagnostic performances of slow-pull suction and standard suction in endoscopic ultrasound-guided fine needle biopsy for gastrointestinal subepithelial tumors. Clin Endosc. 2022; 55:637–644.
Article21. Nabi Z, Reddy DN. Submucosal endoscopy: the present and future. Clin Endosc. 2023; 56:23–37.
Article22. Chen H, Li B, Li L, et al. Current status of endoscopic resection of gastric subepithelial tumors. Am J Gastroenterol. 2019; 114:718–725.
Article23. Lee HL, Kwon OW, Lee KN, et al. Endoscopic histologic diagnosis of gastric GI submucosal tumors via the endoscopic submucosal dissection technique. Gastrointest Endosc. 2011; 74:693–695.
Article24. van Wanrooij RL, Bronswijk M, Kunda R, et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2022; 54:310–332.
Article25. Pimentel-Nunes P, Libânio D, Bastiaansen BA, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline: Update 2022. Endoscopy. 2022; 54:591–622.
Article