Korean J Physiol Pharmacol.
2023 Nov;27(6):521-531. 10.4196/kjpp.2023.27.6.521.
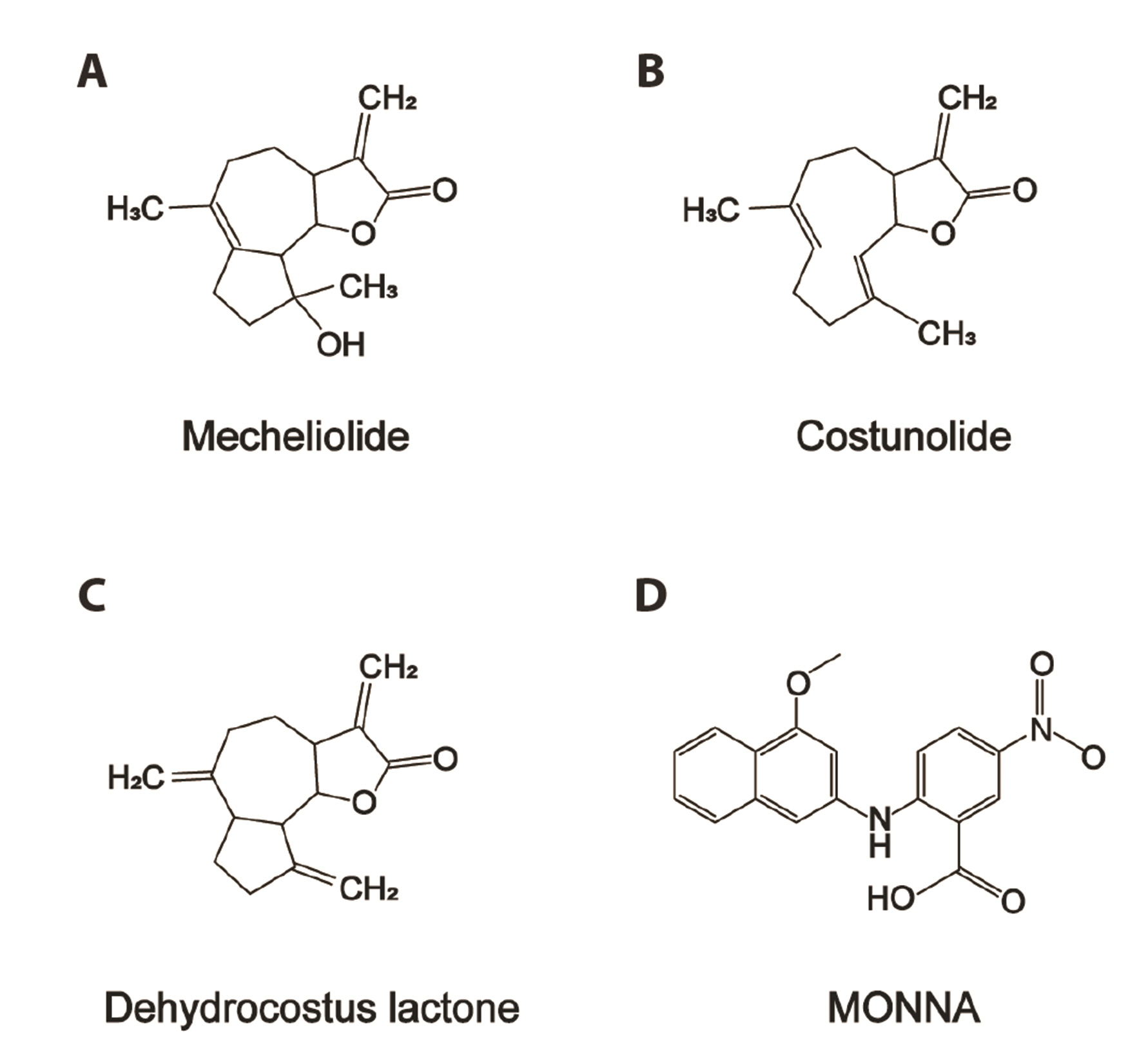
Three sesquiterpene lactones suppress lung adenocarcinoma by blocking TMEM16A-mediated Ca2+ -activated Cl− channels
- Affiliations
-
- 1Department of Pharmacology, Hebei University of Chinese Medicine, Shijiazhuang 050200, China
- 2Hebei International Cooperation Center for Ion Channel Function and Innovative Traditional Chinese Medicine, Shijiazhuang 050091, China
- 3College of Basic Medicine, Hebei University of Chinese Medicine, Shijiazhuang 050200, China
- 4The First Department of Pulmonary and Critical Care Medicine, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, China
- 5Hebei Key Laboratory of Integrative Medicine on Liver-Kidney Patterns, Hebei University of Chinese Medicine, Shijiazhuang 050200, China
- 6Institute of Chinese Integrative Medicine, Hebei Medical University, Shijiazhuang 050017, China
- 7Hebei Higher Education Applied Technology Research Center of TCM Development and Industrialization, Hebei University of Chinese Medicine, Shijiazhuang 050200, China
- 8Department of Pharmacy, Hebei Provincial Hospital of Traditional Chinese Medicine, Shijiazhuang 050000, China
- KMID: 2547318
- DOI: http://doi.org/10.4196/kjpp.2023.27.6.521
Abstract
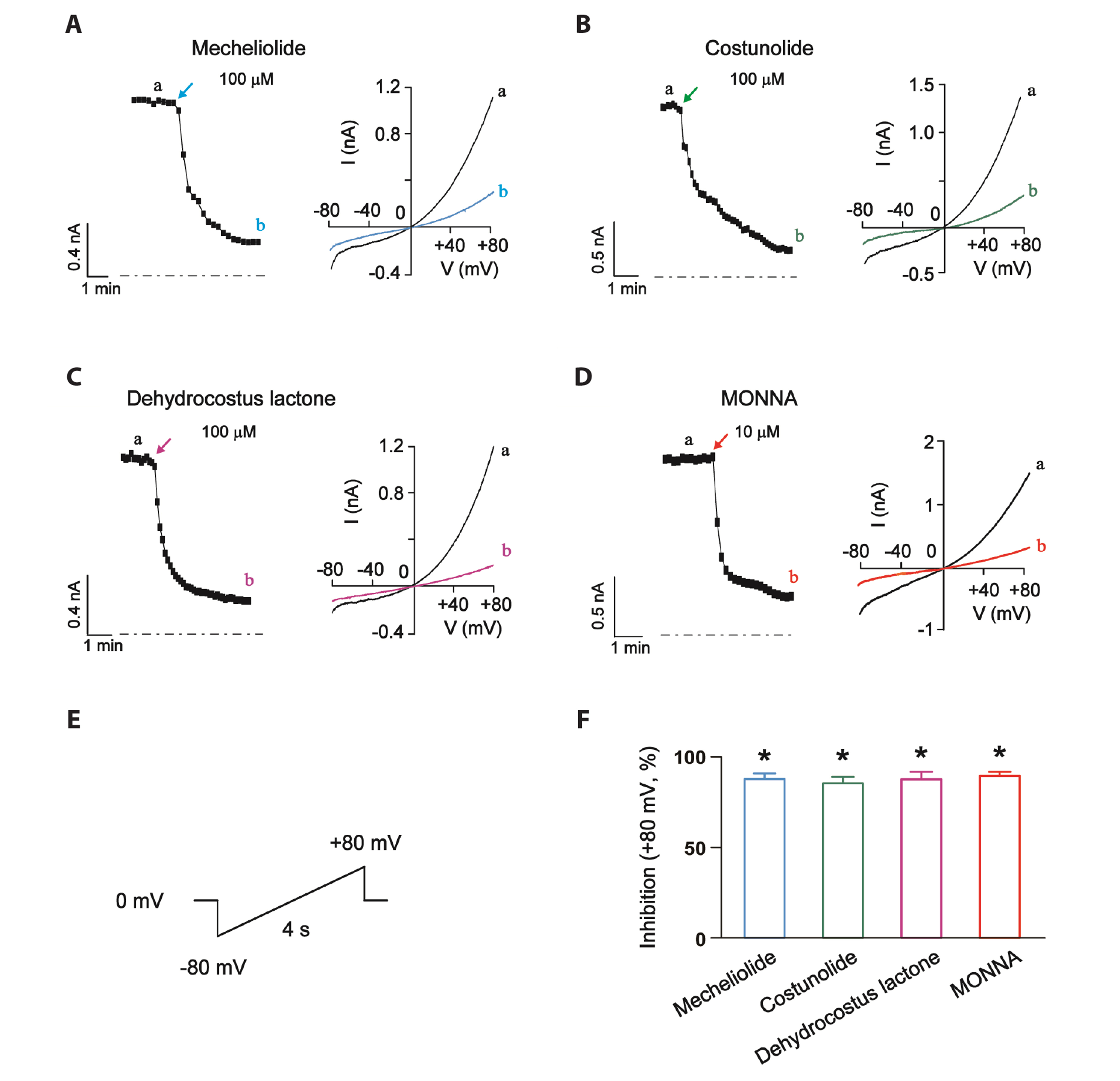
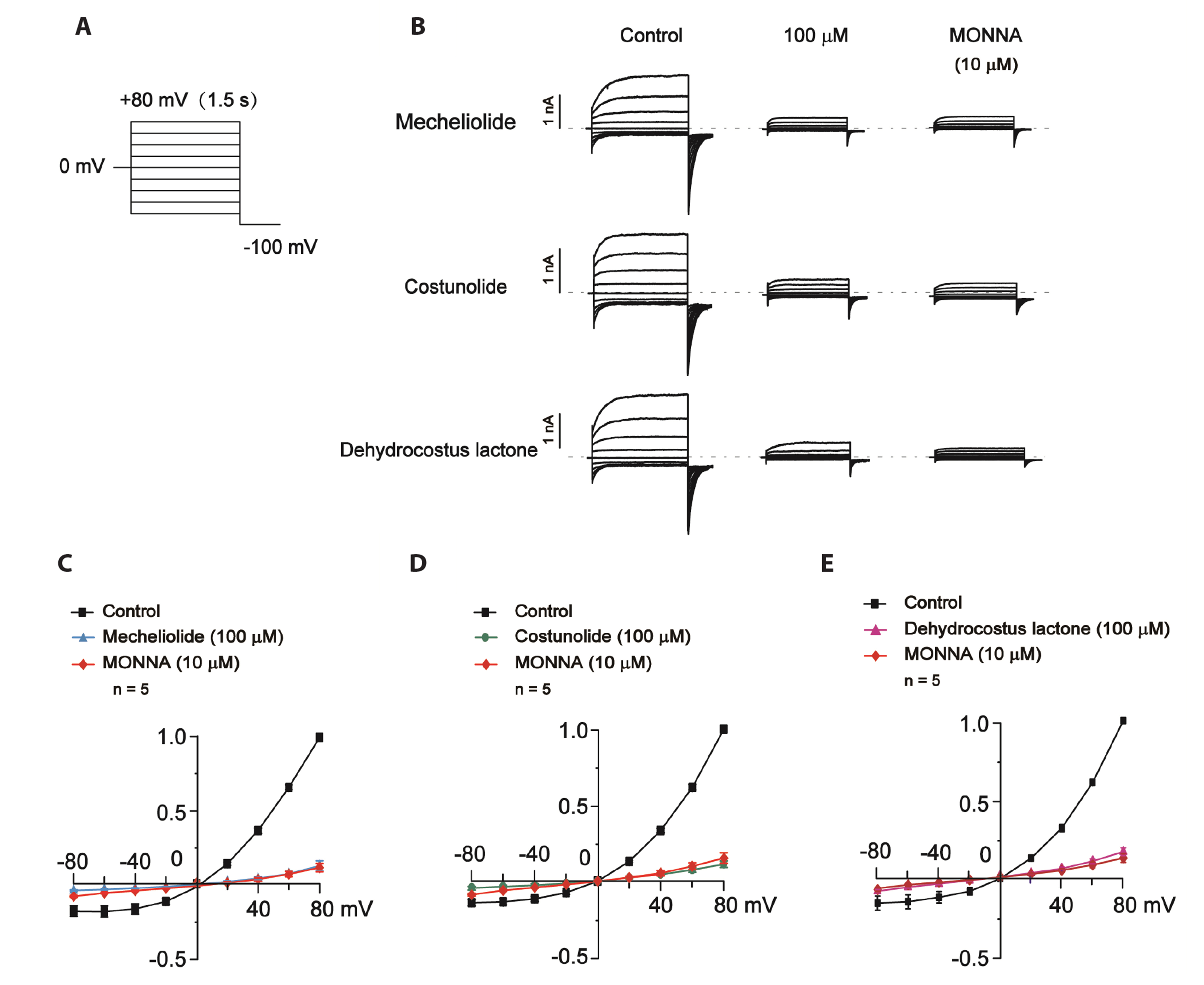
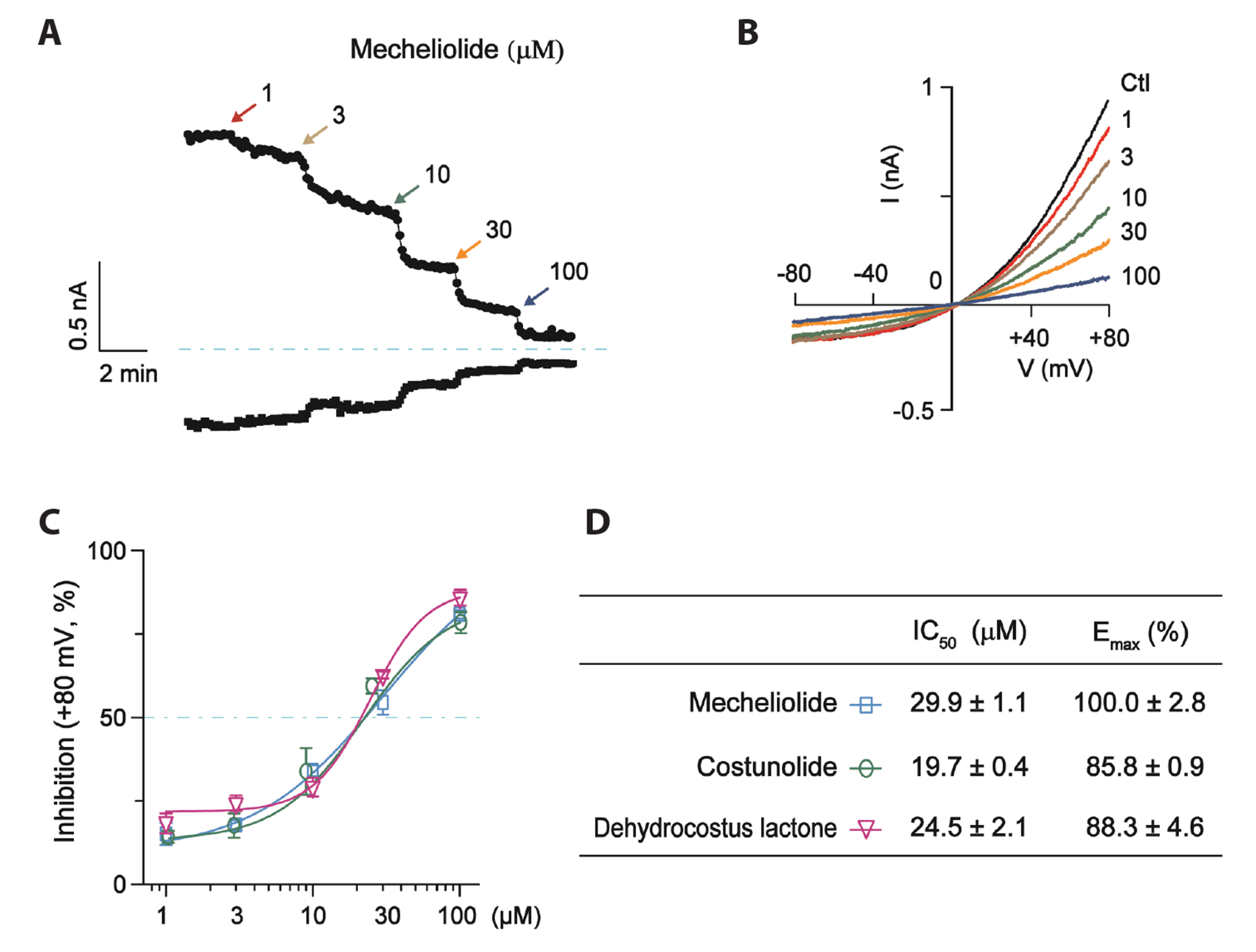
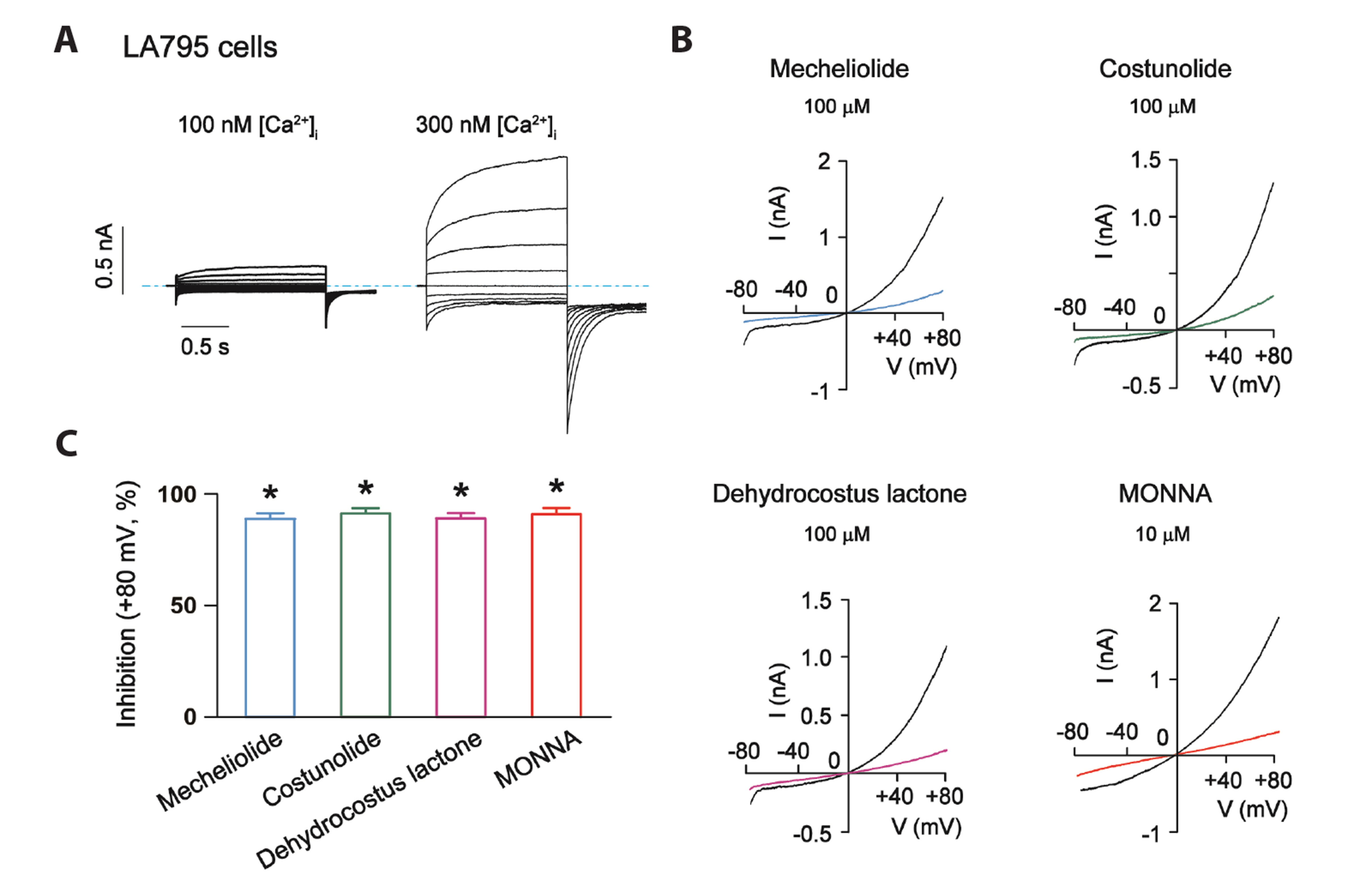
- Transmembrane protein TMEM16A, which encodes calcium-activated chloride channel has been implicated in tumorigenesis. Overexpression of TMEM16A is associated with poor prognosis and low overall survival in multiple cancers including lung adenocarcinoma, making it a promising biomarker and therapeutic target. In this study, three structure-related sesquiterpene lactones (mecheliolide, costunolide and dehydrocostus lactone) were extracted from the traditional Chinese medicine Aucklandiae Radix and identified as novel TMEM16A inhibitors with comparable inhibitory effects. Their effects on the proliferation and migration of lung adenocarcinoma cells were examined. Whole-cell patch clamp experiments showed that these sesquiterpene lactones potently inhibited recombinant TMEM16A currents in a concentration-dependent manner. The half-maximal concentration (IC50 ) values for three tested sesquiterpene lactones were 29.9 ± 1.1 μM, 19.7 ± 0.4 μM, and 24.5 ± 2.1 μM, while the maximal effect (Emax ) values were 100.0% ± 2.8%, 85.8% ± 0.9%, and 88.3% ± 4.6%, respectively. These sesquiterpene lactones also significantly inhibited the endogenous TMEM16A currents and proliferation, and migration of LA795 lung cancer cells. These results demonstrate that mecheliolide, costunolide and dehydrocostus lactone are novel TMEM16A inhibitors and potential candidates for lung adenocarcinoma therapy.
Figure
Reference
-
1. Huang F, Wong X, Jan LY. 2012; International Union of Basic and Clinical Pharmacology. LXXXV: calcium-activated chloride channels. Pharmacol Rev. 64:1–15. DOI: 10.1124/pr.111.005009. PMID: 22090471. PMCID: PMC3250081.
Article2. Scudieri P, Sondo E, Ferrera L, Galietta LJ. 2012; The anoctamin family: TMEM16A and TMEM16B as calcium-activated chloride channels. Exp Physiol. 97:177–183. DOI: 10.1113/expphysiol.2011.058198. PMID: 21984732.
Article3. Huang X, Gollin SM, Raja S, Godfrey TE. 2002; High-resolution mapping of the 11q13 amplicon and identification of a gene, TAOS1, that is amplified and overexpressed in oral cancer cells. Proc Natl Acad Sci U S A. 99:11369–11374. DOI: 10.1073/pnas.172285799. PMID: 12172009. PMCID: PMC123263.
Article4. Katoh M, Katoh M. 2003; FLJ10261 gene, located within the CCND1-EMS1 locus on human chromosome 11q13, encodes the eight-transmembrane protein homologous to C12orf3, C11orf25 and FLJ34272 gene products. Int J Oncol. 22:1375–1381. DOI: 10.3892/ijo.22.6.1375. PMID: 12739008.
Article5. Crottès D, Jan LY. 2019; The multifaceted role of TMEM16A in cancer. Cell Calcium. 82:102050. DOI: 10.1016/j.ceca.2019.06.004. PMID: 31279157. PMCID: PMC6711484.
Article6. Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G, Zhong L, Ma Z, Zheng J, Fang X, Ma T. 2014; Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS One. 9:e115443. DOI: 10.1371/journal.pone.0115443. PMID: 25541940. PMCID: PMC4277312. PMID: e1799d879dad4397a88da7567f62dfe4.
Article7. Shang L, Hao JJ, Zhao XK, He JZ, Shi ZZ, Liu HJ, Wu LF, Jiang YY, Shi F, Yang H, Zhang Y, Liu YZ, Zhang TT, Xu X, Cai Y, Jia XM, Li M, Zhan QM, Li EM, Wang LD, et al. 2016; ANO1 protein as a potential biomarker for esophageal cancer prognosis and precancerous lesion development prediction. Oncotarget. 7:24374–24382. DOI: 10.18632/oncotarget.8223. PMID: 27016410. PMCID: PMC5029708.
Article8. Wang H, Zou L, Ma K, Yu J, Wu H, Wei M, Xiao Q. 2017; Cell-specific mechanisms of TMEM16A Ca2+-activated chloride channel in cancer. Mol Cancer. 16:152. DOI: 10.1186/s12943-017-0720-x. PMID: 28893247. PMCID: PMC5594453. PMID: 98ad363855fd4819a4f8e9fc20e5474f.9. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. Erratum in: CA Cancer J Clin. 2020;70:313. DOI: 10.3322/caac.21492. PMID: 30207593.
Article10. Zhang X, Li H, Zhang H, Liu Y, Huo L, Jia Z, Xue Y, Sun X, Zhang W. 2017; Inhibition of transmembrane member 16A calcium-activated chloride channels by natural flavonoids contributes to flavonoid anticancer effects. Br J Pharmacol. 174:2334–2345. DOI: 10.1111/bph.13841. PMID: 28452066. PMCID: PMC5481650.
Article11. Guo S, Chen Y, Pang C, Wang X, Shi S, Zhang H, An H, Zhan Y. 2019; Matrine is a novel inhibitor of the TMEM16A chloride channel with antilung adenocarcinoma effects. J Cell Physiol. 234:8698–8708. DOI: 10.1002/jcp.27529. PMID: 30370542.
Article12. Guo S, Chen Y, Shi S, Wang X, Zhang H, Zhan Y, An H. 2020; Arctigenin, a novel TMEM16A inhibitor for lung adenocarcinoma therapy. Pharmacol Res. 155:104721. DOI: 10.1016/j.phrs.2020.104721. PMID: 32097750.
Article13. Boedtkjer DM, Kim S, Jensen AB, Matchkov VM, Andersson KE. 2015; New selective inhibitors of calcium-activated chloride channels - T16A(inh) -A01, CaCC(inh) -A01 and MONNA - what do they inhibit? Br J Pharmacol. 172:4158–4172. DOI: 10.1111/bph.13201. PMID: 26013995. PMCID: PMC4543620.
Article14. Seo Y, Lee HK, Park J, Jeon DK, Jo S, Jo M, Namkung W. 2016; Ani9, a novel potent small-molecule ANO1 inhibitor with negligible effect on ANO2. PLoS One. 11:e0155771. DOI: 10.1371/journal.pone.0155771. PMID: 27219012. PMCID: PMC4878759. PMID: 65ccc41fa1db43e88b5ab5b7aca30b52.
Article15. Oh SJ, Hwang SJ, Jung J, Yu K, Kim J, Choi JY, Hartzell HC, Roh EJ, Lee CJ. 2013; MONNA, a potent and selective blocker for transmembrane protein with unknown function 16/anoctamin-1. Mol Pharmacol. 84:726–735. DOI: 10.1124/mol.113.087502. PMID: 23997117. PMCID: PMC3807079.
Article16. Zhang X, Zhang G, Zhao Z, Xiu R, Jia J, Chen P, Liu Y, Wang Y, Yi J. 2021; Cepharanthine, a novel selective ANO1 inhibitor with potential for lung adenocarcinoma therapy. Biochim Biophys Acta Mol Cell Res. 1868:119132. DOI: 10.1016/j.bbamcr.2021.119132. PMID: 34450215.
Article17. Huang Z, Wei C, Yang K, Yu Z, Wang Z, Hu H. 2021; Aucklandiae Radix and Vladimiriae Radix: a systematic review in ethnopharmacology, phytochemistry and pharmacology. J Ethnopharmacol. 280:114372. DOI: 10.1016/j.jep.2021.114372. PMID: 34186101.
Article18. Zhuang K, Xia Q, Zhang S, Maharajan K, Liu K, Zhang Y. 2021; A comprehensive chemical and pharmacological review of three confusable Chinese herbal medicine-Aucklandiae radix, Vladimiriae radix, and Inulae radix. Phytother Res. 35:6655–6689. DOI: 10.1002/ptr.7250. PMID: 34431559.
Article19. Zhang X, Zhang G, Zhai W, Zhao Z, Wang S, Yi J. 2020; Inhibition of TMEM16A Ca2+-activated Cl- channels by avermectins is essential for their anticancer effects. Pharmacol Res. 156:104763. DOI: 10.1016/j.phrs.2020.104763. PMID: 32201246.20. Luo S, Wang H, Bai L, Chen Y, Chen S, Gao K, Wang H, Wu S, Song H, Ma K, Liu M, Yao F, Fang Y, Xiao Q. 2021; Activation of TMEM16A Ca2+-activated Cl- channels by ROCK1/moesin promotes breast cancer metastasis. J Adv Res. 33:253–264. DOI: 10.1016/j.jare.2021.03.005. PMID: 34603794. PMCID: PMC8463928.
Article21. Saberbaghi T, Wong R, Rutka JT, Wang GL, Feng ZP, Sun HS. 2019; Role of Cl- channels in primary brain tumour. Cell Calcium. 81:1–11. DOI: 10.1016/j.ceca.2019.05.004. PMID: 31129471.22. Jia L, Liu W, Guan L, Lu M, Wang K. 2015; Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. PLoS One. 10:e0136584. DOI: 10.1371/journal.pone.0136584. PMID: 26305547. PMCID: PMC4549304. PMID: 7be1e37575d04a47b36c71ff7d0abaff.
Article23. Lee HS, Kim Y. 2020; Aucklandia lappa causes cell wall damage in Candida albicans by reducing chitin and (1,3)-β-D-glucan. J Microbiol Biotechnol. 30:967–973. DOI: 10.4014/jmb.2002.02025. PMID: 32347080. PMCID: PMC9728168.
Article24. Wang W, Li Q, Yan X, Chen Z, Xie Y, Hu H, Wang Z. 2020; Comparative study of raw and processed Vladimiriae Radix on pharmacokinetic and anti-acute gastritis effect through anti-oxidation and anti-inflammation. Phytomedicine. 70:153224. DOI: 10.1016/j.phymed.2020.153224. PMID: 32353684.
Article25. Tastan P, Hajdú Z, Kúsz N, Zupkó I, Sinka I, Kivcak B, Hohmann J. 2019; Sesquiterpene lactones and flavonoids from Psephellus pyrrhoblepharus with antiproliferative activity on human gynecological cancer cell lines. Molecules. 24:3165. DOI: 10.3390/molecules24173165. PMID: 31480332. PMCID: PMC6749316. PMID: 509261a1ef5144a0843d3f582423d903.
Article26. Choi JY, Choi EH, Jung HW, Oh JS, Lee WH, Lee JG, Son JK, Kim Y, Lee SH. 2008; Melanogenesis inhibitory compounds from Saussureae Radix. Arch Pharm Res. 31:294–299. DOI: 10.1007/s12272-001-1154-0. PMID: 18409040.
Article27. Xu Z, Xu J, Sun S, Lin W, Li Y, Lu Q, Li F, Yang Z, Lu Y, Liu W. 2022; Mecheliolide elicits ROS-mediated ERS driven immunogenic cell death in hepatocellular carcinoma. Redox Biol. 54:102351. DOI: 10.1016/j.redox.2022.102351. PMID: 35671636. PMCID: PMC9168183.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular characterisation of pancreatic zymogen granule ion channel and regulator proteins involved in exocytosis
- Spike Frequency Adaptation in Neurons of the Central Nervous System
- Nimodipine as a potential pharmacological tool for characterizing R-type calcium currents
- TMEM16A-Mediated Mucin Secretion in IL-13-Induced Nasal Epithelial Cells From Chronic Rhinosinusitis Patients
- Ca2+ signalling in endothelial cells: Role of ion channels