Korean J Physiol Pharmacol.
2023 Nov;27(6):513-520. 10.4196/kjpp.2023.27.6.513.
Cornuside inhibits glucose-induced proliferation and inflammatory response of mesangial cells
- Affiliations
-
- 1Prevention Medicine, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou 215009,
- 2Nanjing University of Traditional Chinese Medicine, Nanjing 210023,
- 3Department of Endocrinology, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou 215009, China
- 4Cardiovascular Department, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou 215009, China
- 5Respiratory Department, Suzhou TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Suzhou 215009, China
- KMID: 2547317
- DOI: http://doi.org/10.4196/kjpp.2023.27.6.513
Abstract
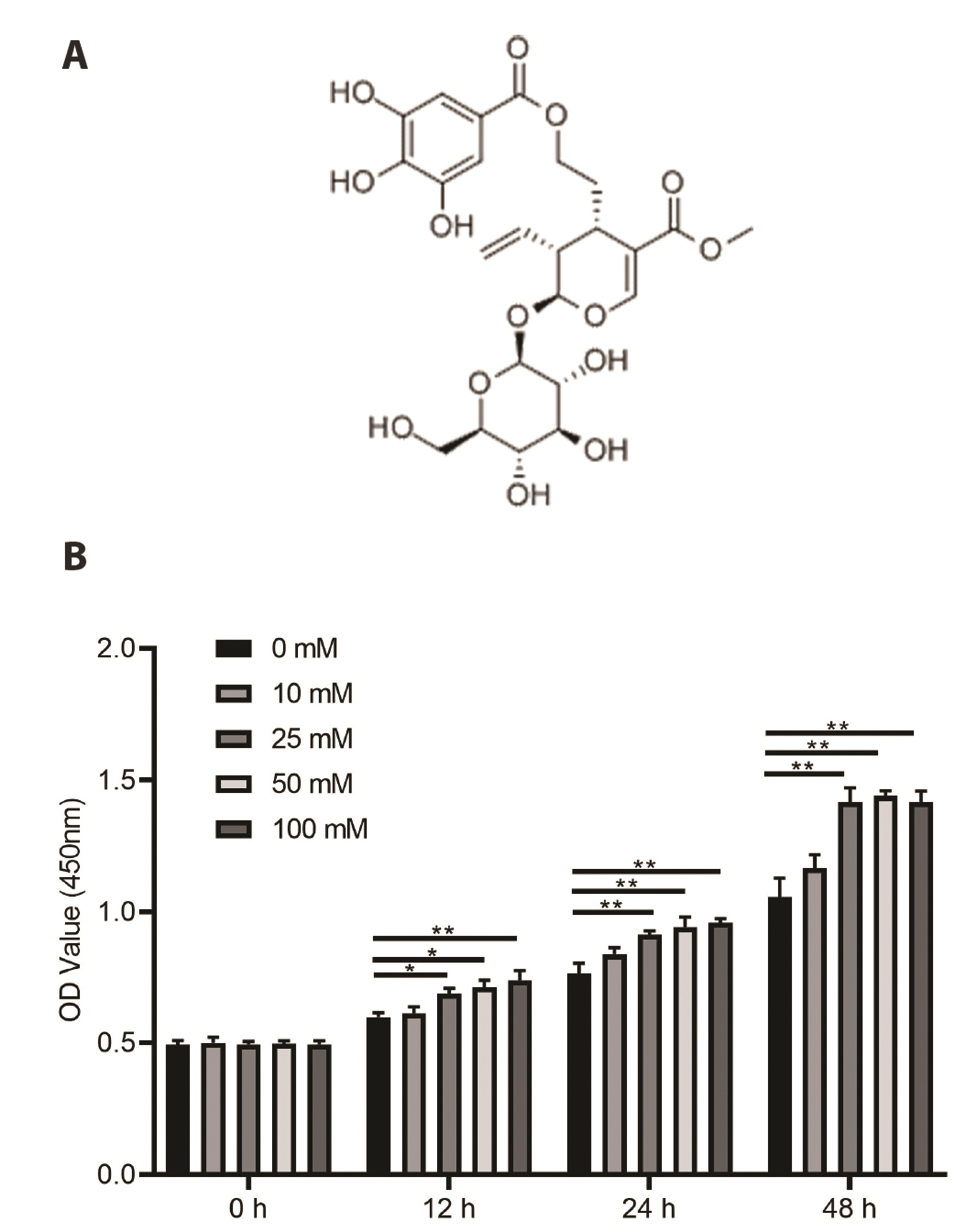
- Cornuside is a secoiridoid glucoside compound extracted from the fruits of Cornus officinalis. Cornuside has immunomodulatory and anti-inflammatory properties; however, its potential therapeutic effects on diabetic nephropathy (DN) have not been completely explored. In this study, we established an in vitro model of DN through treating mesangial cells (MMCs) with glucose. MMCs were then treated with different concentrations of cornuside (0, 5, 10, and 30 μM). Cell viability was determined using cell counting kit-8 and 5-ethynyl-2′-deoxyuridine assays. Levels of proinflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor-α, and IL-1β were examined using enzyme-linked immunosorbent assay. Reverse transcription quantitative real-time polymerase chain reaction and Western blotting were performed to detect the expression of AKT and nuclear factor-kappa B (NF-κB)-associated genes. We found that cornuside treatment significantly reduced glucose-induced increase in MMC viability and expression of pro-inflammatory cytokines. Moreover, cornuside inhibited glucose-induced phosphorylation of AKT and NF-κB inhibitor alpha, decreased the expression of proliferating cell nuclear antigen and cyclin D1, and increased the expression of p21. Our study indicates that the anti-inflammatory properties of cornuside in DN are due to AKT and NF-κB inactivation in MMCs.
Figure
Reference
-
1. Valk EJ, Bruijn JA, Bajema IM. 2011; Diabetic nephropathy in humans: pathologic diversity. Curr Opin Nephrol Hypertens. 20:285–289. DOI: 10.1097/MNH.0b013e328345bc1c. PMID: 21422920.
Article2. Umanath K, Lewis JB. 2018; Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 71:884–895. DOI: 10.1053/j.ajkd.2017.10.026. PMID: 29398179.
Article3. Anders HJ, Huber TB, Isermann B, Schiffer M. 2018; CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 14:361–377. DOI: 10.1038/s41581-018-0001-y. PMID: 29654297.
Article4. Kosmas CE, Silverio D, Sourlas A, Garcia F, Montan PD, Guzman E. 2018; Impact of lipid-lowering therapy on glycemic control and the risk for new-onset diabetes mellitus. Drugs Context. 7:212562. DOI: 10.7573/dic.212562. PMID: 30515229. PMCID: PMC6267678. PMID: 0359c64300c7488ea0c4f4832ef66955.
Article5. A/L B Vasanth Rao VR, Tan SH, Candasamy M, Bhattamisra SK. 2019; Diabetic nephropathy: an update on pathogenesis and drug development. Diabetes Metab Syndr. 13:754–762. DOI: 10.1016/j.dsx.2018.11.054. PMID: 30641802.
Article6. Gao P, Li L, Yang L, Gui D, Zhang J, Han J, Wang J, Wang N, Lu J, Chen S, Hou L, Sun H, Xie L, Zhou J, Peng C, Lu Y, Peng X, Wang C, Miao J, Ozcan U, et al. 2019; Yin Yang 1 protein ameliorates diabetic nephropathy pathology through transcriptional repression of TGFβ1. Sci Transl Med. 11:eaaw2050. DOI: 10.1126/scitranslmed.aaw2050. PMID: 31534017.
Article7. Hotamisligil GS. 2006; Inflammation and metabolic disorders. Nature. 444:860–867. DOI: 10.1038/nature05485. PMID: 17167474.
Article8. Medzhitov R. 2008; Origin and physiological roles of inflammation. Nature. 454:428–435. DOI: 10.1038/nature07201. PMID: 18650913.
Article9. Tuttle KR. 2005; Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol. 16:1537–1538. DOI: 10.1681/ASN.2005040393. PMID: 15872083.
Article10. Li F, Chen Y, Li Y, Huang M, Zhao W. 2020; Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol. 886:173449. DOI: 10.1016/j.ejphar.2020.173449. PMID: 32758570.
Article11. Su Y, Qin W, Wu L, Yang B, Wang Q, Kuang H, Cheng G. 2021; A review of Chinese medicine for the treatment of psoriasis: principles, methods and analysis. Chin Med. 16:138. DOI: 10.1186/s13020-021-00550-y. PMID: 34930402. PMCID: PMC8686297. PMID: 817e6e8dce43484ca4a103f6f54af30f.
Article12. Ryu SH, Kim C, Kim N, Lee W, Bae JS. 2022; Inhibitory functions of cornuside on TGFBIp-mediated septic responses. J Nat Med. 76:451–461. DOI: 10.1007/s11418-021-01601-2. PMID: 35025027. PMCID: PMC8757402.
Article13. Czerwińska ME, Melzig MF. 2018; Cornus mas and Cornus Officinalis-analogies and differences of two medicinal plants traditionally used. Front Pharmacol. 9:894. DOI: 10.3389/fphar.2018.00894. PMID: 30210335. PMCID: PMC6121078. PMID: c372e0d5a3d44654a1df8074afeb019e.14. Ma W, Wang KJ, Cheng CS, Yan GQ, Lu WL, Ge JF, Cheng YX, Li N. 2014; Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J Ethnopharmacol. 153:840–845. DOI: 10.1016/j.jep.2014.03.051. PMID: 24694395.
Article15. Wang Q. 2022; XIST silencing alleviated inflammation and mesangial cells proliferation in diabetic nephropathy by sponging miR-485. Arch Physiol Biochem. 128:1697–1703. DOI: 10.1080/13813455.2020.1789880. PMID: 32669002.
Article16. Saengboonmee C, Phoomak C, Supabphol S, Covington KR, Hampton O, Wongkham C, Gibbs RA, Umezawa K, Seubwai W, Gingras MC, Wongkham S. 2020; NF-κB and STAT3 co-operation enhances high glucose induced aggressiveness of cholangiocarcinoma cells. Life Sci. 262:118548. DOI: 10.1016/j.lfs.2020.118548. PMID: 33038372. PMCID: PMC7686287.
Article17. Gao X, Liu Y, An Z, Ni J. 2021; Active components and pharmacological effects of Cornus officinalis: literature review. Front Pharmacol. 12:633447. DOI: 10.3389/fphar.2021.633447. PMID: 33912050. PMCID: PMC8072387. PMID: ed28052d7152464b8a995551b4bf352d.
Article18. Quah Y, Lee SJ, Lee EB, Birhanu BT, Ali MS, Abbas MA, Boby N, Im ZE, Park SC. 2020; Cornus officinalis ethanolic extract with potential anti-allergic, anti-inflammatory, and antioxidant activities. Nutrients. 12:3317. DOI: 10.3390/nu12113317. PMID: 33138027. PMCID: PMC7692184. PMID: c72cd6fbd95b4edf80cdbcdaede9da3c.
Article19. Fernando PDSM, Piao MJ, Zhen AX, Ahn MJ, Yi JM, Choi YH, Hyun JW. 2020; Extract of Cornus officinalis protects keratinocytes from particulate matter-induced oxidative stress. Int J Med Sci. 17:63–70. DOI: 10.7150/ijms.36476. PMID: 31929739. PMCID: PMC6945560.
Article20. Gao F, Xia SL, Wang XH, Zhou XX, Wang J. 2021; Cornuside I promoted osteogenic differentiation of bone mesenchymal stem cells through PI3K/Akt signaling pathway. J Orthop Surg Res. 16:397. DOI: 10.1186/s13018-021-02508-0. PMID: 34154621. PMCID: PMC8218506. PMID: 6079757930e44346b3144cfd44c97e99.
Article21. Li L, Jin G, Jiang J, Zheng M, Jin Y, Lin Z, Li G, Choi Y, Yan G. 2016; Cornuside inhibits mast cell-mediated allergic response by down-regulating MAPK and NF-κB signaling pathways. Biochem Biophys Res Commun. 473:408–414. DOI: 10.1016/j.bbrc.2016.03.007. PMID: 26972254.
Article22. Kang DG, Moon MK, Lee AS, Kwon TO, Kim JS, Lee HS. 2007; Cornuside suppresses cytokine-induced proinflammatory and adhesion molecules in the human umbilical vein endothelial cells. Biol Pharm Bull. 30:1796–1799. DOI: 10.1248/bpb.30.1796. PMID: 17827743.
Article23. Zhang R, Liu J, Xu B, Wu Y, Liang S, Yuan Q. 2021; Cornuside alleviates experimental autoimmune encephalomyelitis by inhibiting Th17 cell infiltration into the central nervous system. J Zhejiang Univ Sci B. 22:421–430. DOI: 10.1631/jzus.B2000771. PMID: 33973423. PMCID: PMC8110462.
Article24. Choi YH, Jin GY, Li GZ, Yan GH. 2011; Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol Pharm Bull. 34:959–966. DOI: 10.1248/bpb.34.959. PMID: 21719998.
Article25. Wada J, Makino H. 2013; Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152. DOI: 10.1042/CS20120198. PMID: 23075333.
Article26. Yu H, Lin L, Zhang Z, Zhang H, Hu H. 2020; Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 5:209. DOI: 10.1038/s41392-020-00312-6. PMID: 32958760. PMCID: PMC7506548.
Article27. Yi H, Peng R, Zhang LY, Sun Y, Peng HM, Liu HD, Yu LJ, Li AL, Zhang YJ, Jiang WH, Zhang Z. 2017; LincRNA-Gm4419 knockdown ameliorates NF-κB/NLRP3 inflammasome-mediated inflammation in diabetic nephropathy. Cell Death Dis. 8:e2583. DOI: 10.1038/cddis.2016.451. PMID: 28151474. PMCID: PMC5386454.
Article28. Wu L, Liu C, Chang DY, Zhan R, Sun J, Cui SH, Eddy S, Nair V, Tanner E, Brosius FC, Looker HC, Nelson RG, Kretzler M, Wang JC, Xu M, Ju W, Zhao MH, Chen M, Zheng L. 2021; Annexin A1 alleviates kidney injury by promoting the resolution of inflammation in diabetic nephropathy. Kidney Int. 100:107–121. Erratum in: Kidney Int. 2021;100:1349-1350. DOI: 10.1016/j.kint.2021.02.025. PMID: 33675846. PMCID: PMC8893600.
Article29. Zhao Y, Zhang W, Jia Q, Feng Z, Guo J, Han X, Liu Y, Shang H, Wang Y, Liu WJ. 2018; High dose vitamin E attenuates diabetic nephropathy via alleviation of autophagic stress. Front Physiol. 9:1939. DOI: 10.3389/fphys.2018.01939. PMID: 30719008. PMCID: PMC6348272. PMID: d7fb718cd2e14e8297b2897e2877bf73.
Article30. Sun Z, Ma Y, Chen F, Wang S, Chen B, Shi J. 2018; Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem Biol Interact. 293:11–19. DOI: 10.1016/j.cbi.2018.07.011. PMID: 30031708.
Article31. Sun X, Chen L, He Z. 2019; PI3K/Akt-Nrf2 and anti-inflammation effect of macrolides in chronic obstructive pulmonary disease. Curr Drug Metab. 20:301–304. DOI: 10.2174/1389200220666190227224748. PMID: 30827233.
Article32. Xu Z, Jia K, Wang H, Gao F, Zhao S, Li F, Hao J. 2021; METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial-mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell Death Dis. 12:32. DOI: 10.1038/s41419-020-03312-0. PMID: 33414476. PMCID: PMC7791055. PMID: 958ea652b9b94c8e833522db485802c6.
Article33. Hou B, Li Y, Li X, Zhang C, Zhao Z, Chen Q, Zhang N, Li H. 2020; HGF protected against diabetic nephropathy via autophagy-lysosome pathway in podocyte by modulating PI3K/Akt-GSK3β-TFEB axis. Cell Signal. 75:109744. DOI: 10.1016/j.cellsig.2020.109744. PMID: 32827692.
Article34. Lou Z, Li Q, Wang C, Li Y. 2022; The effects of microRNA-126 reduced inflammation and apoptosis of diabetic nephropathy through PI3K/AKT signalling pathway by VEGF. Arch Physiol Biochem. 128:1265–1274. DOI: 10.1080/13813455.2020.1767146. PMID: 32449863.
Article35. Rai U, Kosuru R, Prakash S, Tiwari V, Singh S. 2019; Tetramethylpyrazine alleviates diabetic nephropathy through the activation of Akt signalling pathway in rats. Eur J Pharmacol. 865:172763. DOI: 10.1016/j.ejphar.2019.172763. PMID: 31682792.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corrigendum to: Cornuside inhibits glucose-induced proliferation and inflammatory response of mesangial cells
- The effects of high glucose concentration on the synthesis of extracellular matrix and the fine structures of mesangial cells in culture
- The effects of high glucose concentration on phospholipase A2 activity in cultured rat mesangial cells
- Caveolin-1 is involved in high glucose accelerated human glomerular mesangial cell senescence
- Insulin Enhances Suppressive Effect of Lipopolysaccharide on Glucose-induced Proliferation of Vascular Smooth Muscle Cells