J Korean Med Sci.
2023 Oct;38(41):e341. 10.3346/jkms.2023.38.e341.
De Novo ANCA-Negative Pauci-Immune Crescentic Glomerulonephritis After COVID-19 mRNA Vaccination: A Case Report
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea
- 2Hankook Renal Pathology Lab, Seoul, Korea
- 3Division of Nephrology, Department of Internal Medicine, Chung-Ang University Gwangmyeong Hospital, Gwangmyeong, Korea
- 4Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2547179
- DOI: http://doi.org/10.3346/jkms.2023.38.e341
Abstract
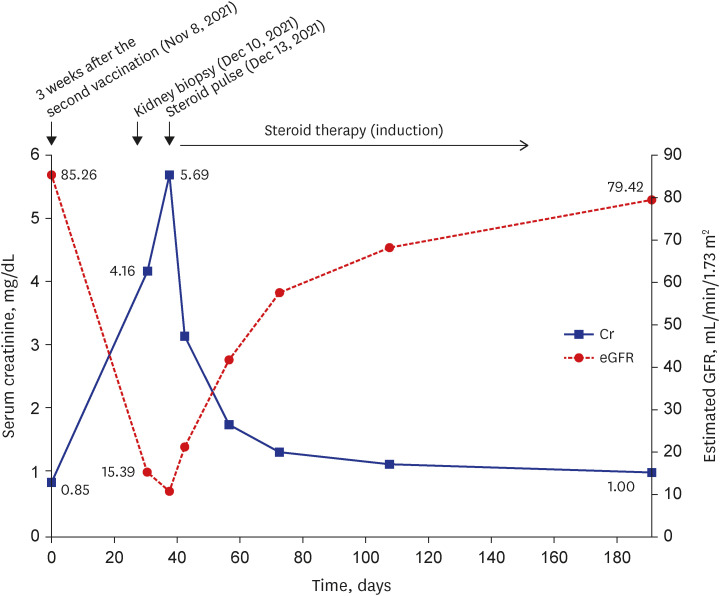
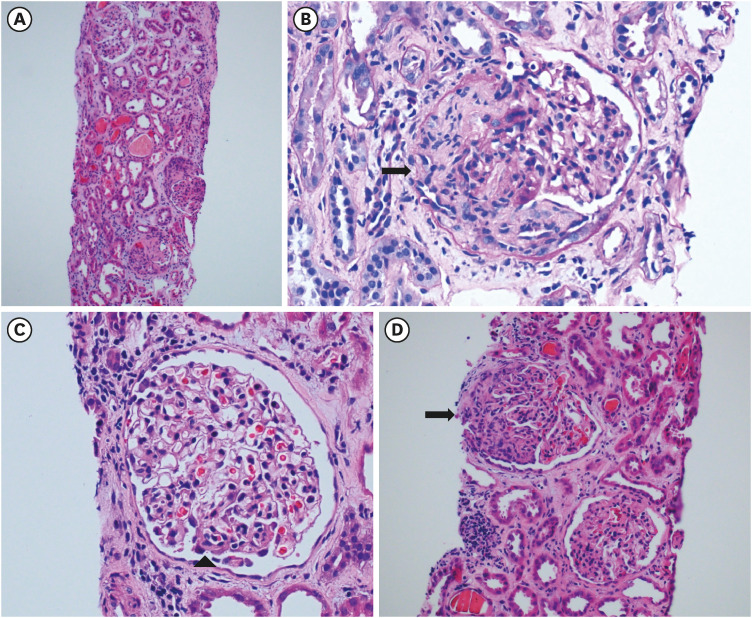
- To prevent the spread of the coronavirus disease 2019 (COVID-19) pandemic, vaccines have been authorized for emergency use and implemented worldwide. We present a case of de novo glomerulonephritis (GN) after use of the COVID-19 mRNA vaccine BNT162b2. A 48-year-old man with no relevant medical history was referred for sudden and persistent worsening of renal insufficiency 1.5 months after the second vaccine dose. He had arthralgia and skin rash a week after vaccination. Abdominal pain and diarrhea started 2 weeks later, and he was admitted to the hospital for enteritis treatment. Colonoscopy showed multiple ulcerations and petechiae suggestive of vasculitis in the terminal ileum. After prednisolone therapy, his gastrointestinal symptoms improved, but his renal function continued to deteriorate. Based on kidney biopsy findings and nephrotic-range proteinuria (5,306 mg/24 hours), he was diagnosed with anti-neutrophil cytoplasmic autoantibody (ANCA)-negative pauci-immune crescentic GN (CrGN). He received high-dose steroid pulse therapy and oral cyclophosphamide, and then, gradually underwent steroid tapering, with improvement in proteinuria and renal function over several weeks. Several cases of GN suspected to be related to COVID-19 vaccines have been reported. To our knowledge, this is the first case report of ANCA-negative pauci-immune crescentic CrGN with extrarenal involvement after COVID-19 mRNA vaccination. Our finding expands the spectrum of COVID-19 vaccine-associated GN.
Keyword
Figure
Reference
-
1. Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021; 385(10):875–884. PMID: 34233097.2. Wagenhäuser I, Reusch J, Gabel A, Krone LB, Kurzai O, Petri N, et al. Bivalent BNT162b2 mRNA original/omicron BA.4-5 booster vaccination: adverse reactions and inability to work compared with the monovalent COVID-19 booster. Clin Microbiol Infect. 2023; 29(4):554–556. PMID: 36657489.3. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.4. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.5. Jeffs LS, Nitschke J, Tervaert JW, Peh CA, Hurtado PR. Viral RNA in the influenza vaccine may have contributed to the development of ANCA-associated vasculitis in a patient following immunisation. Clin Rheumatol. 2016; 35(4):943–951. PMID: 26361945.6. Caza TN, Cassol CA, Messias N, Hannoudi A, Haun RS, Walker PD, et al. Glomerular disease in temporal association with SARS-CoV-2 vaccination: a series of 29 cases. Kidney360. 2021; 2(11):1770–1780. PMID: 35372991.7. Klomjit N, Alexander MP, Fervenza FC, Zoghby Z, Garg A, Hogan MC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep. 2021; 6(12):2969–2978. PMID: 34632166.8. Lim CA, Lee HS, Yoon S, Kim EJ, Seo JW, Koo JR, et al. Focal segmental glomerulosclerosis following the Pfizer-BioNTech COVID-19 vaccine. Kidney Res Clin Pract. 2022; 41(2):263–266. PMID: 35286791.9. Park JS, Lee EY. Renal side effects of COVID-19 vaccines in patients with immunoglobulin A nephropathy. Kidney Res Clin Pract. 2022; 41(1):124–127. PMID: 35108771.10. El Hasbani G, Uthman I. ANCA-associated vasculitis following the first dose of Pfizer-BioNTech COVID-19 vaccine. Nephron. 2023; 147(2):103–107. PMID: 35850104.11. Kim BC, Kim HS, Han KH, Han SY, Jo HA. A case report of MPO-ANCA-associated vasculitis following heterologous mRNA1273 COVID-19 booster vaccination. J Korean Med Sci. 2022; 37(26):e204. PMID: 35790206.12. Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021; 78(4):611–613. PMID: 34280507.13. So D, Min KW, Jung WY, Han SW, Yu MY. Microscopic polyangiitis following mRNA COVID-19 vaccination: a case report. J Korean Med Sci. 2022; 37(19):e154. PMID: 35578586.14. Waldman M, Sinaii N, Lerma EV, Kurien AA, Jhaveri KD, Uppal NN, et al. COVID-19 vaccination and new onset glomerular disease: results from the IRocGN2 international registry. Kidney360. 2023; 4(3):349–362. PMID: 36996301.15. Kim S, Jung J, Cho H, Lee J, Go H, Lee JH. A child with crescentic glomerulonephritis following SARS-CoV-2 mRNA (Pfizer-BioNTech) vaccination. Pediatr Nephrol. 2023; 38(1):299–302. PMID: 35854121.16. Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, et al. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant. 2005; 20(7):1392–1399. PMID: 15855209.17. Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY. Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol. 2007; 18(2):599–605. PMID: 17215440.18. Ronsin C, Georges M, Chapelet-Debout A, Augusto JF, Audard V, Lebourg L, et al. ANCA-negative pauci-immune necrotizing glomerulonephritis: a case series and a new clinical classification. Am J Kidney Dis. 2022; 79(1):56–68.e1. PMID: 34119564.19. Chen M, Kallenberg CG, Zhao MH. ANCA-negative pauci-immune crescentic glomerulonephritis. Nat Rev Nephrol. 2009; 5(6):313–318. PMID: 19399019.20. Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int. 2021; 100(5):959–965. PMID: 34534551.21. Cagigi A, Loré K. Immune responses induced by mRNA vaccination in mice, monkeys and humans. Vaccines (Basel). 2021; 9(1):61. PMID: 33477534.22. Turner JS, O’Halloran JA, Kalaidina E, Kim W, Schmitz AJ, Zhou JQ, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021; 596(7870):109–113. PMID: 34182569.23. Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol. 2020; 65:14–20. PMID: 32244193.24. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020; 217:108480. PMID: 32461193.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- De Novo Crescentic Glomerulonephritis Following COVID-19 Infection: A Pediatric Case Report
- A Case of ANCA-associated Pauci-immune Crescentic Glomerulonephritis in Juvenile Rheumatoid Arthritis
- A Case of P-ANCA Positive Necrotizing Glomerulonephritis with Eosinophilia
- A Case Report of MPO-ANCAAssociated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination
- A Case of Crescentic Glomerulonephritis with Coexisting Anti-Glomerular Basement Membrane Antibodies and Myeloperoxidase-Anti-Neutrophil Cytoplasmic Autoantibodies