Ann Surg Treat Res.
2023 Oct;105(4):228-236. 10.4174/astr.2023.105.4.228.
Evaluation of the efficacy and safety of conversion from the tacrolimus capsule to tablet in stable liver transplant recipients with maintenance therapy: a 24-week, open-label, single-center, phase IV exploratory clinical study
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2546785
- DOI: http://doi.org/10.4174/astr.2023.105.4.228
Abstract
- Purpose
The tablet form of tacrolimus is more convenient for drug ingestion than the capsule form. We examined the efficacy and safety of tacrolimus tablets and a satisfaction survey after formula conversion in liver transplant (LT) recipients.
Methods
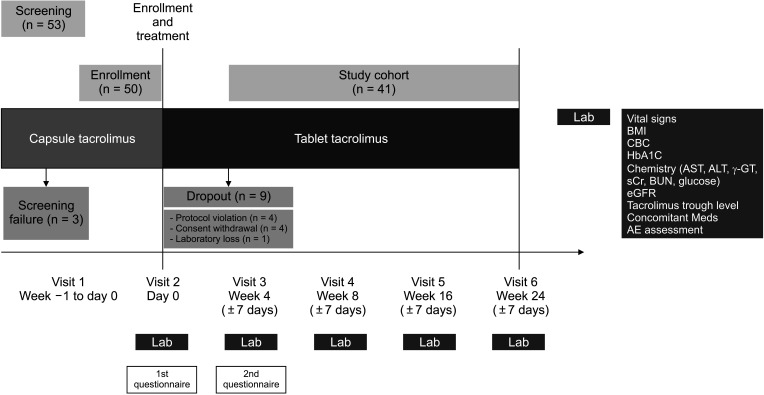
This study was an open-label, prospective clinical trial for tacrolimus formula 1:1 conversion from capsule to tablet in 41 adult LT recipients with tacrolimus maintenance therapy of more than 1 month. The primary endpoint was incidence of biopsy-proven acute rejection (BPAR) within 24 weeks. Surveys 1 week before and 4 weeks after formula conversion were conducted for total daily dose of medication, number, scale of discomfort and satisfaction.
Results
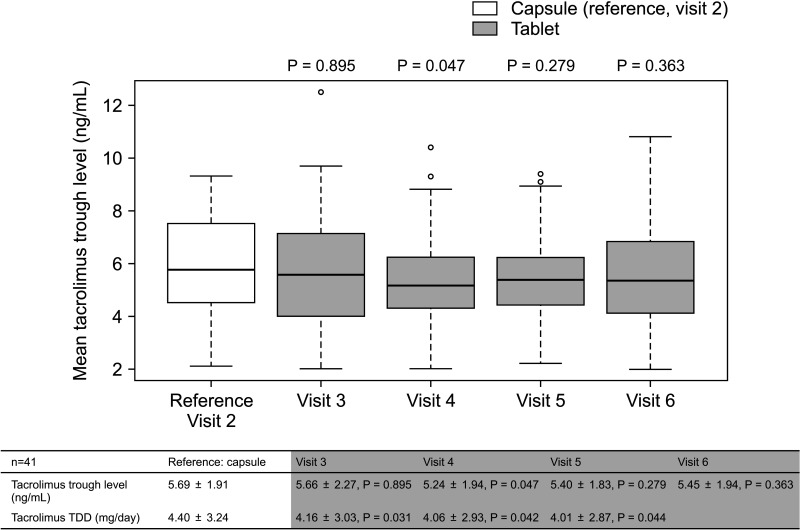
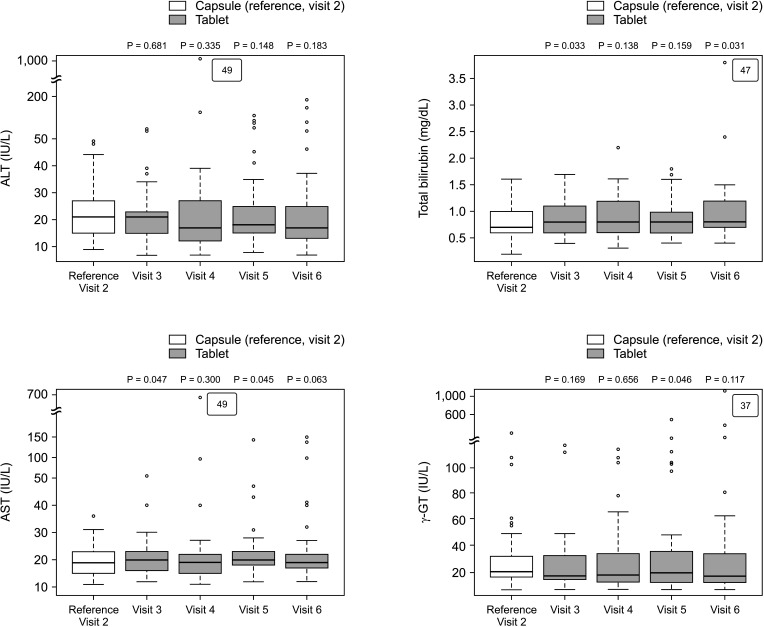
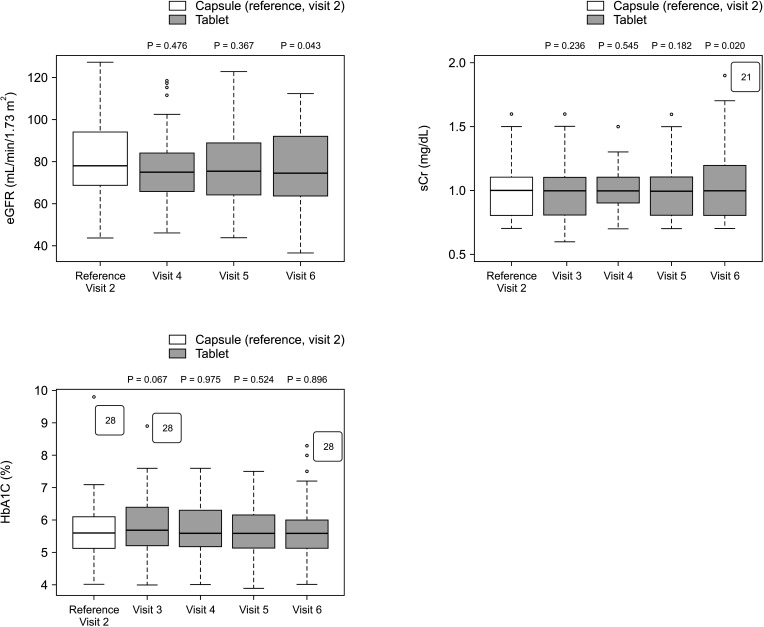
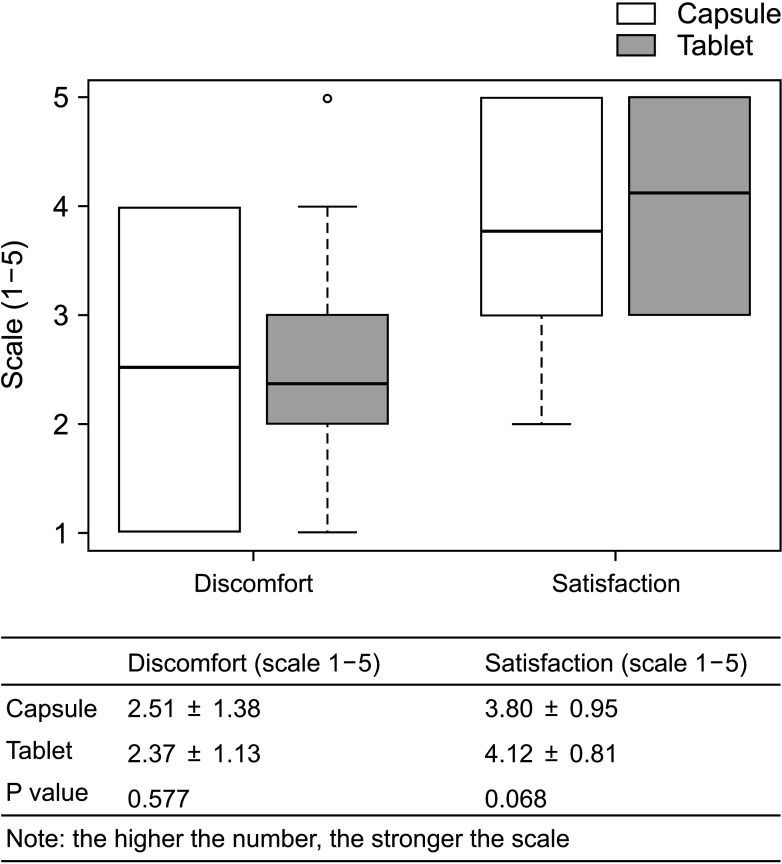
The overall incidence of BPAR was 0% and there was no graft loss or patient death. The incidence of adverse effects was 34.1% (n = 14) after formula conversion. The most common severe adverse effect was abnormal liver function test (n = 5): biliary complications (n = 4) and alcoholic recidivism (n = 1). Total daily dose and number of tacrolimus doses were significantly lower after formula conversion (P < 0.05) without changes in trough level. According to survey analysis, there was no significant difference in discomfort and satisfaction scales from capsule to tablet conversion (P < 0.05).
Conclusion
The present study suggests that the new tablet formula can be a useful treatment option to maintain a consistent level of tacrolimus with a lower total daily dose and number in adult LT recipients.
Figure
Reference
-
1. Goralczyk AD, Bari N, Abu-Ajaj W, Lorf T, Ramadori G, Friede T, et al. Calcineurin inhibitor sparing with mycophenolate mofetil in liver transplantion: a systematic review of randomized controlled trials. Am J Transplant. 2012; 12:2601–2607. PMID: 22813081.
Article2. Jain AB, Hamad I, Rakela J, Dodson F, Kramer D, Demetris J, et al. A prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone, and mycophenolate mofetil in primary adult liver transplant recipients: an interim report. Transplantation. 1998; 66:1395–1398. PMID: 9846530.
Article3. Kim H, Yi NJ, Lee J, Kim J, Moon MR, Jeong J, et al. Safety of reduced dose of mycophenolate mofetil combined with tacrolimus in living-donor liver transplantation. Clin Mol Hepatol. 2014; 20:291–299. PMID: 25320733.
Article4. McAlister VC, Haddad E, Renouf E, Malthaner RA, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant. 2006; 6:1578–1585. PMID: 16827858.
Article5. Burra P, Germani G, Gnoato F, Lazzaro S, Russo FP, Cillo U, et al. Adherence in liver transplant recipients. Liver Transpl. 2011; 17:760–770. PMID: 21384527.
Article6. Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the 'ReSpECT' study. Am J Transplant. 2009; 9:327–336. PMID: 19120077.
Article7. TruneČka P, Klempnauer J, Bechstein WO, Pirenne J, Friman S, Zhao A, et al. Renal function in de novo liver transplant recipients receiving different prolonged-release tacrolimus regimens: the DIAMOND study. Am J Transplant. 2015; 15:1843–1854. PMID: 25707487.
Article8. An International Panel. Demetris AJ, Batts KP, Dhillon AP, Ferrell L, Fung J, et al. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997; 25:658–663. PMID: 9049215.
Article9. Kim YK, Kim A, Park SJ, Lee H. New tablet formulation of tacrolimus with smaller interindividual variability may become a better treatment option than the conventional capsule formulation in organ transplant patients. Drug Des Devel Ther. 2017; 11:2861–2869.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Single Center Experiences of Conversion from Twice-daily Tacrolimus (Prograf) to Once-daily Tacrolimus (Advagraf) in Stable Liver Transplant Recipients
- Efficacy and safety of prolonged-release versus immediate-release tacrolimus in de novo liver transplant recipients in South Korea: a randomized open-label phase 4 study (MAPLE)
- Modified Release Tacrolimus
- Safety and efficacy of conversion to once-daily tacrolimus from twice-daily tacrolimus in pediatric liver transplant recipients
- Efficacy and safety of a switch from twicedaily tacrolimus to once-daily generic tacrolimus in stable liver transplant patients