J Korean Med Sci.
2023 Sep;38(37):e292. 10.3346/jkms.2023.38.e292.
Clinical Utility of Sero-Immunological Responses Against SARS-CoV-2 Nucleocapsid Protein During Subsequent Prevalence of Wild-Type, Delta Variant, and Omicron Variant
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Infectious Diseases, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Asia Pacific Foundation for Infectious Diseases (APFID), Seoul, Korea
- KMID: 2546202
- DOI: http://doi.org/10.3346/jkms.2023.38.e292
Abstract
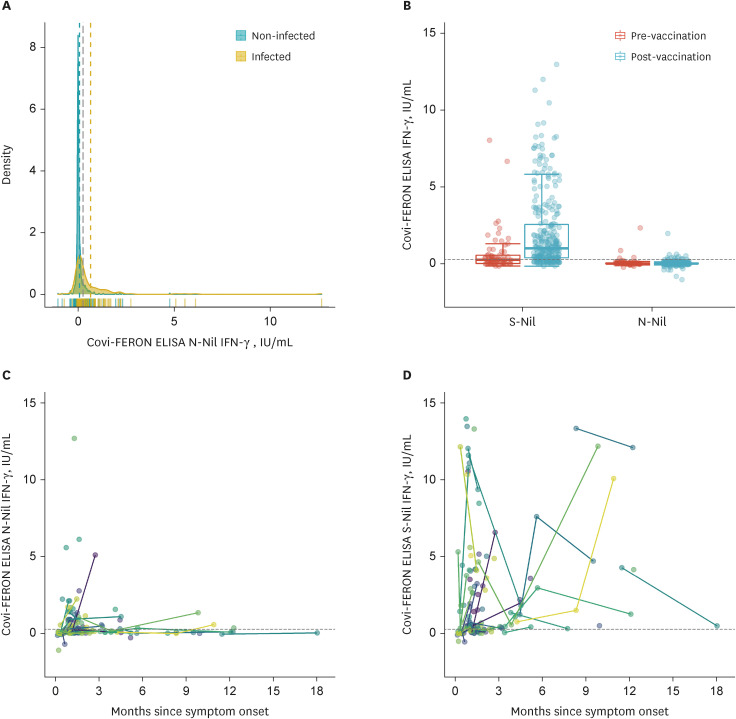
- As nucleocapsid protein of severe acute respiratory syndrome coronavirus 2 is immunogenic but not targeted in vaccines, it could be useful in distinguishing natural infection from vaccination. We aimed to investigate the clinical utility of sero-immunological responses against the nucleocapsid protein. Nucleocapsid antibody immunoassay study with 302 coronavirus disease 2019 (COVID-19) patients showed lower titers in immunocompromised patients (P < 0.001), higher titers in higher severity (P = 0.031), and different seroconversion rates and titers according to variants of concern. Longitudinal evaluation of nucleocapsid antibodies using 513 samples from 291 COVID-19 patients revealed that it could persist up to 556 days from symptom onset. Interferon gamma release assay against the nucleocapsid protein showed poor response, precluding the deduction of a cut-off for the nucleocapsid protein. In conclusion, nucleocapsid antibody provides instructive clues about the immunogenicity of nucleocapsid proteins by different seroconversion rates and titers according to the severity of infection, host immune status, and different variants of concern.
Keyword
Figure
Reference
-
1. Ko JH, Joo EJ, Kim SH, Kim YJ, Huh K, Cho SY, et al. Clinical application of rapid diagnostic test kit for SARS-CoV-2 antibodies into the field of patient care. J Microbiol Immunol Infect. 2021; 54(1):97–100. PMID: 32684340.
Article2. Ko JH, Joo EJ, Baek JY, Huh K, Cho SY, Kang CI, et al. Evaluation of six anti-SARS-CoV-2 antibody test kits and practical approaches to optimize the diagnostic performance. J Microbiol Immunol Infect. 2021; 54(5):983–986. PMID: 33836943.
Article3. Lee B, Ko JH, Park J, Moon HW, Baek JY, Jung S, et al. Estimating the neutralizing effect and titer correlation of semi-quantitative anti-SARS-CoV-2 antibody immunoassays. Front Cell Infect Microbiol. 2022; 12:822599. PMID: 35493733.
Article4. Ko JH, Lee JY, Kim HA, Kang SJ, Baek JY, Park SJ, et al. Serologic evaluation of healthcare workers caring for COVID-19 patients in the Republic of Korea. Front Microbiol. 2020; 11:587613. PMID: 33329460.
Article5. Yang J, Ko JH, Baek JY, Hong J, Ha S, Lee B, et al. Effects of short-term corticosteroid use on reactogenicity and immunogenicity of the first dose of ChAdOx1 nCoV-19 vaccine. Front Immunol. 2021; 12:744206. PMID: 34630425.
Article6. Ko JH, Joo EJ, Park SJ, Baek JY, Kim WD, Jee J, et al. Neutralizing antibody production in asymptomatic and mild COVID-19 patients, in comparison with pneumonic COVID-19 patients. J Clin Med. 2020; 9(7):2268. PMID: 32708872.
Article7. Lee B, Ko JH, Lee KH, Kim YC, Song YG, Park YS, et al. Estimation of SARS-CoV-2 neutralizing activity and protective immunity in different vaccine types using three surrogate virus neutralization test assays and two semiquantitative binding assays targeting the receptor-binding domain. Microbiol Spectr. 2022; 10(6):e0266922. PMID: 36250875.
Article8. Bae S, Ko JH, Choi JY, Park WJ, Lim SY, Ahn JY, et al. Heterologous ChAdOx1 and Bnt162b2 vaccination induces strong neutralizing antibody responses against SARS-CoV-2 including delta variant with tolerable reactogenicity. Clin Microbiol Infect. 2022; 28(10):1390.e1–1390.e7.
Article9. Data Analysis Team, Epidemiological Investigation and Analysis Task Force, Central Disease Control Headquarters, Korea Disease Control and Prevention Agency (KDCA). Outbreak report of COVID-19 during designation of class 1 infectious disease in the Republic of Korea (January 20, 2020–April 24, 2022). Updated 2023. Accessed August 8, 2022. https://www.kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=720267&act=view .10. Yang J, Won G, Baek JY, Lee YH, Kim H, Huh K, et al. Neutralizing activity against Omicron BA.5 after tixagevimab/cilgavimab administration comparable to those after Omicron BA.1/BA.2 breakthrough infections. Front Immunol. 2023; 14:1139980. PMID: 36936968.
Article11. Johnson BA, Zhou Y, Lokugamage KG, Vu MN, Bopp N, Crocquet-Valdes PA, et al. Nucleocapsid mutations in SARS-CoV-2 augment replication and pathogenesis. PLoS Pathog. 2022; 18(6):e1010627. PMID: 35728038.
Article12. Wang P, Liu L, Nair MS, Yin MT, Luo Y, Wang Q, et al. SARS-CoV-2 neutralizing antibody responses are more robust in patients with severe disease. Emerg Microbes Infect. 2020; 9(1):2091–2093. PMID: 32930052.
Article13. Yang L, Xu Q, Yang B, Li J, Dong R, Da J, et al. IgG antibody titers against SARS-CoV-2 nucleocapsid protein correlate with the severity of COVID-19 patients. BMC Microbiol. 2021; 21(1):351. PMID: 34922455.
Article14. Song SH, Chung KY, Jee Y, Chung HS, Kim K, Minn D, et al. Immunogenicity of SARS-CoV-2 vaccine in kidney transplant recipients: a cross-sectional study in Korea. J Korean Med Sci. 2023; 38(5):e22. PMID: 36747360.
Article15. Oh YJ, Kim J, Kang ES, Rhu J, Choi GS, Joh JW. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients: a single-center study. J Korean Med Sci. 2023; 38(16):e121. PMID: 37096307.
Article16. Le Bert N, Tan AT, Kunasegaran K, Tham CY, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020; 584(7821):457–462. PMID: 32668444.
Article17. Alfego D, Sullivan A, Poirier B, Williams J, Adcock D, Letovsky S. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine. 2021; 36:100902. PMID: 34056568.
Article18. Krutikov M, Palmer T, Tut G, Fuller C, Azmi B, Giddings R, et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev. 2022; 3(1):e13–e21. PMID: 34935001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant proteins of spike protein of SARS-CoV-2 with the Omicron receptor-binding domain induce production of highly Omicron-specific neutralizing antibodies
- Kinetics of Neutralizing Antibody Responses Against SARS-CoV-2 Delta Variant in Patients Infected at the Beginning of the Pandemic
- SARS-CoV-2 Omicron Variant of Concern: Everything You Wanted to Know about Omicron but Were Afraid to Ask
- Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea
- Increased viral load in patients infected with severe acute respiratory syndrome coronavirus 2 Omicron variant in the Republic of Korea