Kosin Med J.
2023 Sep;38(3):201-209. 10.7180/kmj.23.127.
Post-percutaneous core needle biopsy sputum cytology: diagnostic value and factors for positive prediction in diagnosing malignancy
- Affiliations
-
- 1Department of Radiology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Department of Pathology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- KMID: 2546155
- DOI: http://doi.org/10.7180/kmj.23.127
Abstract
- Background
This study evaluated the diagnostic yield and positive predictive factors of post-percutaneous core needle biopsy (PCNB) sputum cytology in diagnosing malignancy.
Methods
This retrospective study included patients who underwent PCNB at a single center from January 2014 to March 2022. Patient demographics, lung lesion characteristics on computed tomography, underlying lung disease, post-PCNB complications, histopathologic results of PCNB, post-PCNB sputum specimens, and final diagnoses were reviewed. The diagnostic yields and related factors were analyzed.
Results
Overall, 177 consecutive patients with sputum specimens obtained after PCNB for intrapulmonary lesions were enrolled. Among them, 152 patients had a final diagnosis of malignancy. Diagnostic sputum specimens with atypical or malignant cells were obtained in 12 patients. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of sputum cytology were 7.89%, 100%, 20.90%, 100%, and 15.15%, respectively. Lesion size, air-bronchogram, lesion multiplicity, and the cell type of squamous cell and adenocarcinoma differed significantly between the groups with diagnostic versus non-diagnostic sputum (p<0.05). The lesion size (odds ratio [OR], 1.035; 95% confidence interval [CI], 1.008–1.064; p=0.013), presence of air-bronchogram (OR, 23.485; 95% CI, 2.532–217.316; p=0.005), and squamous cell carcinoma (OR, 7.397; 95% CI, 1.773–30.865; p=0.006) were significantly associated with a diagnostic sputum specimen post-PCNB.
Conclusions
Although post-PCNB sputum cytology had low sensitivity in diagnosing lung cancer, it showed diagnostic results in some peripheral lung cancer patients who have squamous cell types, relatively large tumors, and air-bronchograms in the lesions.
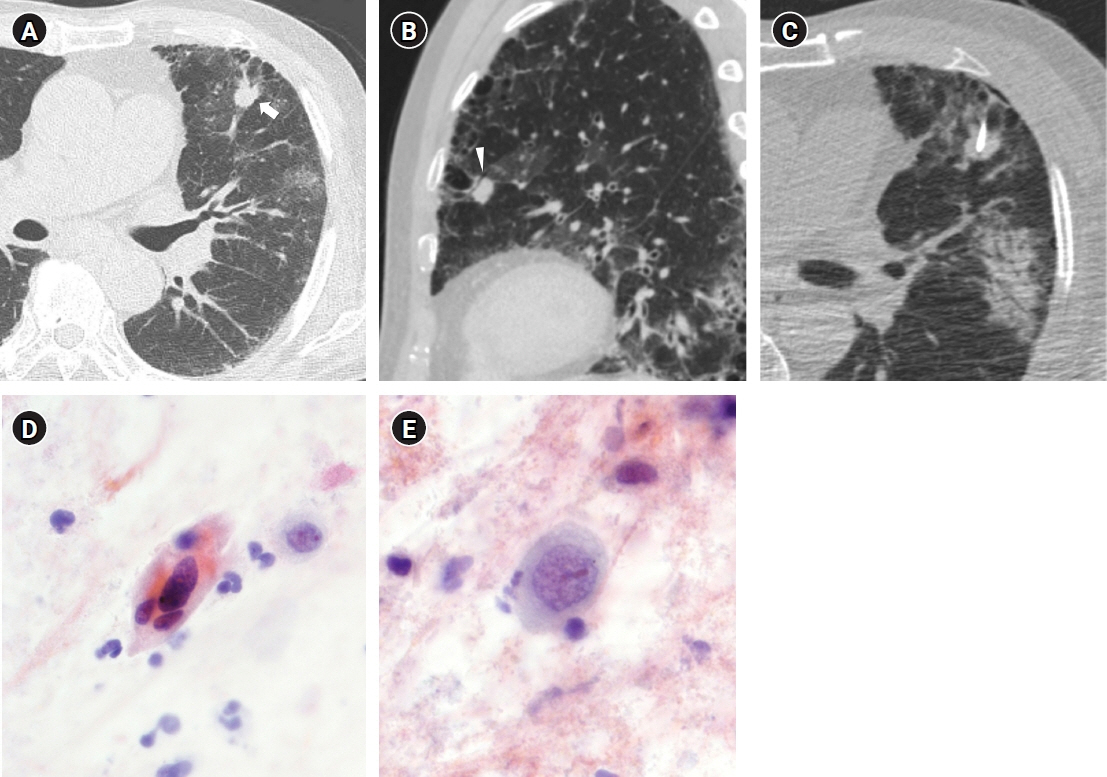
Figure
Reference
-
References
1. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021; 49:778–89.
Article2. Kang MJ, Won YJ, Lee JJ, Jung KW, Kim HJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2019. Cancer Res Treat. 2022; 54:330–44.
Article3. Flehinger BJ, Melamed MR, Zaman MB, Heelan RT, Perchick WB, Martini N. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Memorial Sloan-Kettering study. Am Rev Respir Dis. 1984; 130:555–60.4. Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984; 130:561–5.5. Frost JK, Ball WC Jr, Levin ML, Tockman MS, Baker RR, Carter D, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis. 1984; 130:549–54.6. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.
Article7. Diederich S, Wormanns D. Impact of low-dose CT on lung cancer screening. Lung Cancer. 2004; 45 Suppl 2:S13–9.
Article8. Hubers AJ, Prinsen CF, Sozzi G, Witte BI, Thunnissen E. Molecular sputum analysis for the diagnosis of lung cancer. Br J Cancer. 2013; 109:530–7.
Article9. Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003; 123(1 Suppl):115S–128S.10. Sing A, Freudenberg N, Kortsik C, Wertzel H, Klosa B, Hasse J. Comparison of the sensitivity of sputum and brush cytology in the diagnosis of lung carcinomas. Acta Cytol. 1997; 41:399–408.
Article11. Chae KJ, Hong H, Yoon SH, Hahn S, Jin GY, Park CM, et al. Non-diagnostic results of percutaneous transthoracic needle biopsy: a meta-analysis. Sci Rep. 2019; 9:12428.
Article12. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung AN, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology. 2017; 284:228–43.
Article13. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008; 246:697–722.
Article14. Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018; 198:e44–68.
Article15. Yun S, Kang H, Park S, Kim BS, Park JG, Jung MJ. Diagnostic accuracy and complications of CT-guided core needle lung biopsy of solid and part-solid lesions. Br J Radiol. 2018; 91:20170946.
Article16. Yamagami T, Yoshimatsu R, Miura H, Yamada K, Takahata A, Matsumoto T, et al. Diagnostic performance of percutaneous lung biopsy using automated biopsy needles under CT-fluoroscopic guidance for ground-glass opacity lesions. Br J Radiol. 2013; 86:20120447.
Article17. Lee KH, Lim KY, Suh YJ, Hur J, Han DH, Kang MJ, et al. Nondiagnostic percutaneous transthoracic needle biopsy of lung lesions: a multicenter study of malignancy risk. Radiology. 2019; 290:814–23.
Article18. Neumann T, Meyer M, Patten FW, Johnson FL, Erozan YS, Frable WJ, et al. Premalignant and malignant cells in sputum from lung cancer patients. Cancer. 2009; 117:473–81.
Article19. Bocking A, Biesterfeld S, Chatelain R, Gien-Gerlach G, Esser E. Diagnosis of bronchial carcinoma on sections of paraffin-embedded sputum. Sensitivity and specificity of an alternative to routine cytology. Acta Cytol. 1992; 36:37–47.20. Risse EK, van't Hof MA, Vooijs GP. Relationship between patient characteristics and the sputum cytologic diagnosis of lung cancer. Acta Cytol. 1987; 31:159–65.21. Hirsch FR, Franklin WA, Gazdar AF, Bunn PA Jr. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res. 2001; 7:5–22.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of Fine Needle Aspiration Cytology of Bone Lesions
- Usefulness of Core Needle Biopsy Compared with Fine Needle Aspiration Cytology for Palpable Breast Masses
- Accuracy of Core Needle Biopsy Versus Fine Needle Aspiration Cytology for Diagnosing Salivary Gland Tumors
- Percutaneous Fine Needle Aspiration Biopsy of Lung Masses
- Percutaneous Needle Aspiration Biopsy of Chest Lesions: Effectivenese When Using an 18-Gauge Needle