J Korean Med Sci.
2023 Sep;38(35):e272. 10.3346/jkms.2023.38.e272.
Real-World Effectiveness of Nirmatrelvir-Ritonavir and Its Acceptability in High-Risk COVID-19 Patients
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, National Medical Center, Seoul, Korea
- 2Public Health Research Institute, National Medical Center, Seoul, Korea
- 3Seoul Veterans Hospital Medical Center, Seoul, Korea
- 4Department of Pediatrics, National Medical Center, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea
- 6Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, National Medical Center, Seoul, Korea
- 7National Institute of Infectious Diseases, Korea National Institute of Health, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 8Division of Infectious Diseases, Department of Internal Medicine, Seoul Medical Center, Seoul, Korea
- KMID: 2545551
- DOI: http://doi.org/10.3346/jkms.2023.38.e272
Abstract
- Background
Nirmatrelvir-ritonavir is highly effective in preventing severe coronavirus disease 2019 (COVID-19) in high-risk patients with mild-to-moderate severity. However, real-world performance data are limited, and the drug is not so acceptable to the COVID-19 patients at high risk who need it in Korea.
Methods
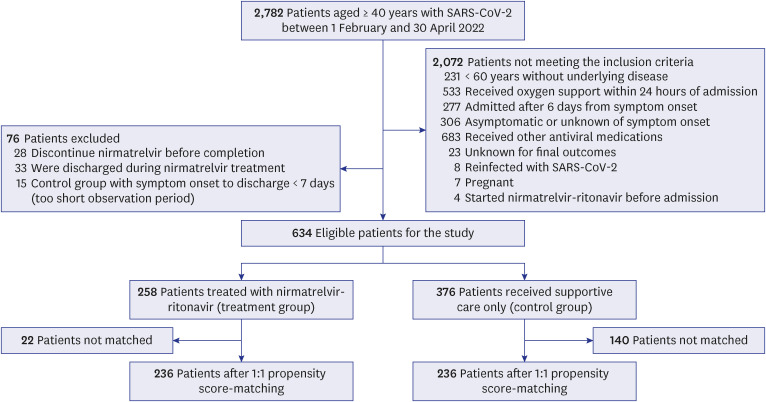
To evaluate the effectiveness of nirmatrelvir-ritonavir, we conducted a propensity score-matched retrospective cohort study on patients with mild-to-moderate COVID-19 at high risk for a severe disease who were hospitalized at four hospitals in South Korea from February 2022 to April 2022. A total of 236 patients in the treatment group (administered nirmatrelvir-ritonavir) and 236 in the matched control group (supportive care only) were analyzed for the primary outcome, i.e., the time to oxygen support-free survival. The secondary outcome was a composite result of disease progression. The reason for not prescribing nirmatrelvir-ritonavir to the indicated patients was also investigated.
Results
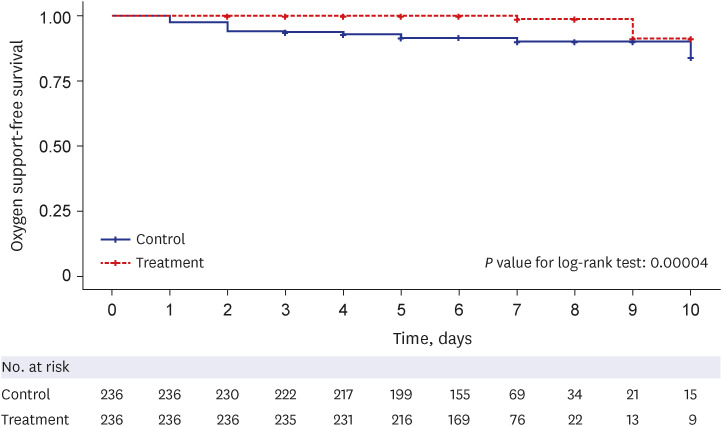
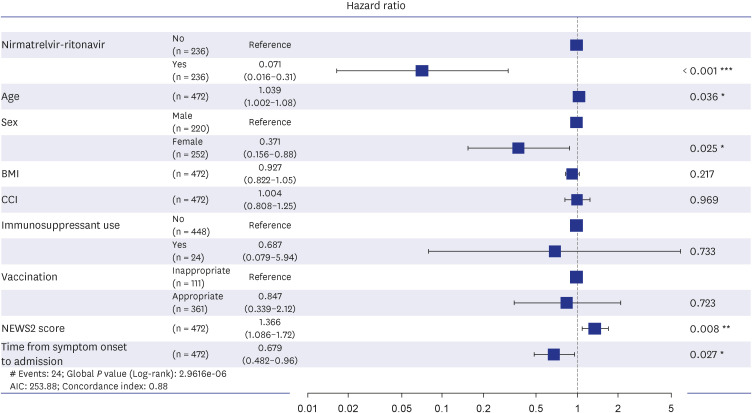
The treatment group showed significantly longer oxygen support-free survival than the matched control group (adjusted hazard ratio [aHR], 0.07; 95% confidence interval [CI], 0.01–0.31; P < 0.001). Multivariate Cox regression analysis showed that age (aHR, 1.03; 95% CI, 1.00–1.07), National Early Warning Score-2 at admission (aHR, 1.36; 95% CI, 1.08–1.71), nirmatrelvir-ritonavir treatment, female sex (aHR, 0.37; 95% CI, 0.15–0.88), and time from symptom onset to admission (aHR, 0.67; 95% CI, 0.48–0.95) were significantly associated with oxygen therapy. However, none of the factors were related to the composite outcome. In the unmatched control group, 19.9% of 376 patients had documented explanations for nirmatrelvir-ritonavir non-prescription, and 44.0% of these were due to contraindication criteria. In the treatment group, 10.9% of patients discontinued the medication primarily because of adverse events (71.4%), with gastrointestinal symptoms being the most common (50.0%).
Conclusion
Nirmatrelvir-ritonavir treatment significantly reduced oxygen therapy requirements in high-risk patients with COVID-19 during the omicron variant surge in South Korea. Physicians are encouraged to consider the active use of nirmatrelvir-ritonavir and to be watchful for gastrointestinal symptoms during medication.
Figure
Reference
-
1. Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021; 374(6575):1586–1593. PMID: 34726479.
Article2. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. Updated December 22, 2021. Accessed March 5, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 .3. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022; 386(15):1397–1408. PMID: 35172054.
Article4. Hung YP, Lee JC, Chiu CW, Lee CC, Tsai PJ, Hsu IL, et al. Oral nirmatrelvir/ritonavir therapy for COVID-19: the dawn in the dark? Antibiotics (Basel). 2022; 11(2):220. PMID: 35203821.
Article5. Korea Disease Control and Prevention Agency. Press release: COVID-19 vaccination and outbreak situation. Updated February 1, 2022. Accessed February 26, 2023. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=718518&cg_code=&act=view&nPage=84 .6. Kang SH, Kim SW, Kim AY, Cho KH, Park JW, Do JY. Association between chronic kidney disease or acute kidney injury and clinical outcomes in COVID-19 Patients. J Korean Med Sci. 2020; 35(50):e434. PMID: 33372426.
Article7. Kim L, Garg S, O’Halloran A, Whitaker M, Pham H, Anderson EJ, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis. 2021; 72(9):e206–e214. PMID: 32674114.
Article8. Ko JY, Danielson ML, Town M, Derado G, Greenlund KJ, Kirley PD, et al. Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021; 72(11):e695–e703. PMID: 32945846.
Article9. Sung HK, Kim JY, Heo J, Seo H, Jang YS, Kim H, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January–May 2020. J Korean Med Sci. 2020; 35(30):e280. PMID: 32743995.
Article10. Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION Network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(4):139–145. PMID: 35085224.
Article11. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int. 2016; 113(35-36):597–603. PMID: 27658473.
Article12. Yuan Y, Yung YF, Stokes M. Propensity score methods for causal inference with the PSMATCH procedure. Proceedings of the SAS® Global Forum 2017 Conference, Lake Buena Vista, FL, USA. 2017; Paper SAS332-2017. Updated 2017. Accessed March 6, 2023. https://support.sas.com/resources/papers/proceedings17/SAS0332-2017.pdf .13. Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009; 51(1):171–184. PMID: 19197955.
Article14. Kassambara A, Kosinski M, Biecek P, Fabian S. Package ‘survminer.’ Drawing survival curves using “ggplot2” (R package version 03 1). Updated 2017. Accessed March 5, 2023. https://cran.microsoft.com/snapshot/2017-04-21/web/packages/survminer/survminer.pdf .15. Therneau TM, Lumley T. Package ‘Survival’. R Top Doc. 2015; 128(10):28–33.16. Park H, Park YJ, Lee HY, Yu M, Song YJ, Lee SE, et al. The effectiveness of Paxlovid treatment in long-term care facilities in South Korea during the outbreak of the Omicron variant of SARS-CoV-2. Osong Public Health Res Perspect. 2022; 13(6):443–447. PMID: 36617550.
Article17. Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N Engl J Med. 2022; 387(9):790–798. PMID: 36001529.
Article18. Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023; 76(3):e342–e349. PMID: 35653428.
Article19. Wong CK, Au IC, Lau KT, Lau EH, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022; 22(12):1681–1693. PMID: 36029795.
Article20. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020; 11(1):29. PMID: 32450906.
Article21. Pérez-López FR, Tajada M, Savirón-Cornudella R, Sánchez-Prieto M, Chedraui P, Terán E. Coronavirus disease 2019 and gender-related mortality in European countries: a meta-analysis. Maturitas. 2020; 141:59–62. PMID: 33036704.
Article22. Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020; 116(14):2197–2206. PMID: 33063089.
Article23. Tartof SY, Slezak JM, Puzniak L, Hong V, Xie F, Ackerson BK, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case-control study. Lancet Respir Med. 2022; 10(7):689–699. PMID: 35468336.
Article24. Korea Disease Control and Prevention Agency. Press release. Updated August 16, 2022. Accessed February 26, 2023. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=720451&cg_code=&act=view&nPage=37 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determining and Comparing the RealWorld Effectiveness of Molnupiravir and Nirmatrelvir-Ritonavir in Patients Hospitalized With COVID-19
- Nirmatrelvir/Ritonavir Prescription Rate and Outcomes in Coronavirus Disease 2019: A Single Center Study
- Effectiveness and Adverse Events of Nirmatrelvir/Ritonavir Versus Molnupiravir for COVID-19 in Outpatient Setting: Multicenter Prospective Observational Study
- Effect of Paxlovid in COVID-19 treatment during the periods of SARS-CoV-2 Omicron BA.5 and BN.1 subvariant dominance in the Republic of Korea: a retrospective cohort study
- Treatment Options for Patients With Mild-to-Moderate Coronavirus Disease 2019 in Korea