Endocrinol Metab.
2023 Aug;38(4):395-405. 10.3803/EnM.2023.1661.
Phloretin Ameliorates Succinate-Induced Liver Fibrosis by Regulating Hepatic Stellate Cells
- Affiliations
-
- 1Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Physiology, Keimyung University School of Medicine, Daegu, Korea
- KMID: 2545272
- DOI: http://doi.org/10.3803/EnM.2023.1661
Abstract
- Background
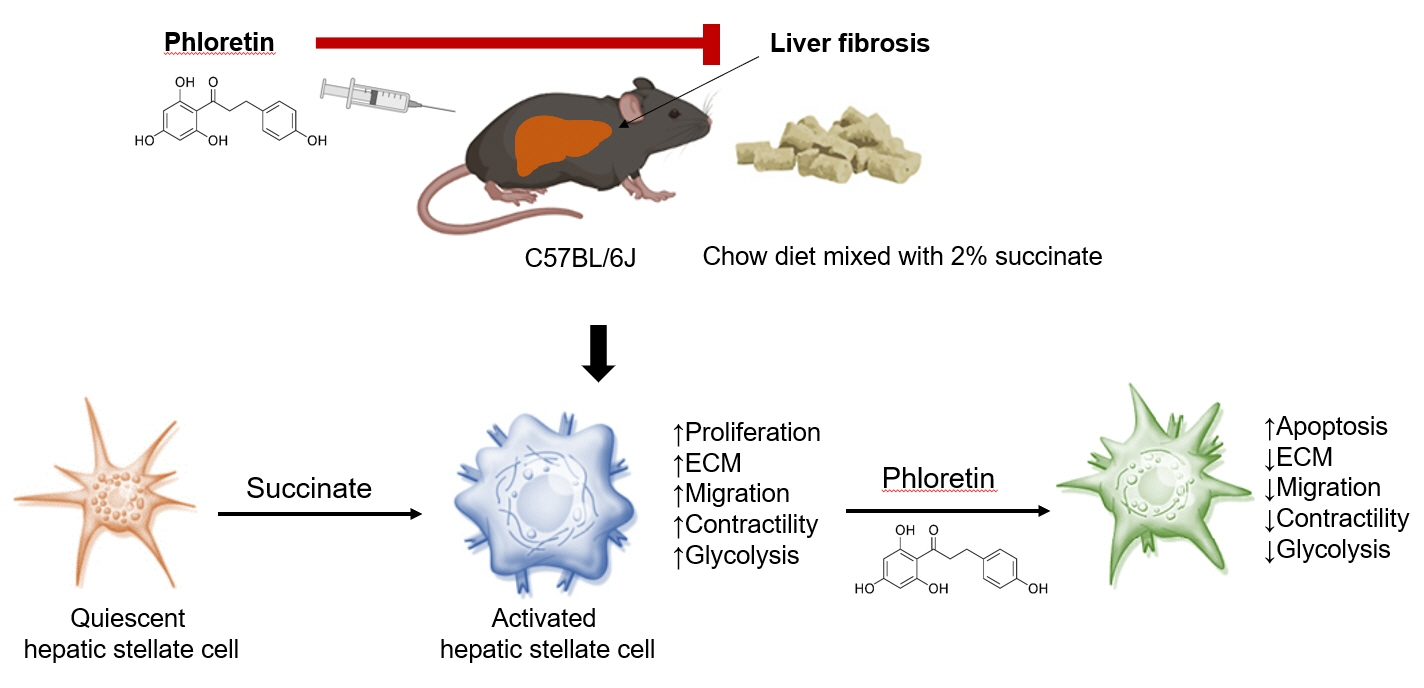
Hepatic stellate cells (HSCs) are the major cells which play a pivotal role in liver fibrosis. During injury, extracellular stimulators can induce HSCs transdifferentiated into active form. Phloretin showed its ability to protect the liver from injury, so in this research we would like to investigate the effect of phloretin on succinate-induced HSCs activation in vitro and liver fibrosis in vivo study.
Methods
In in vitro, succinate was used to induce HSCs activation, and then the effect of phloretin on activated HSCs was examined. In in vivo, succinate was used to generated liver fibrosis in mouse and phloretin co-treated to check its protection on the liver.
Results
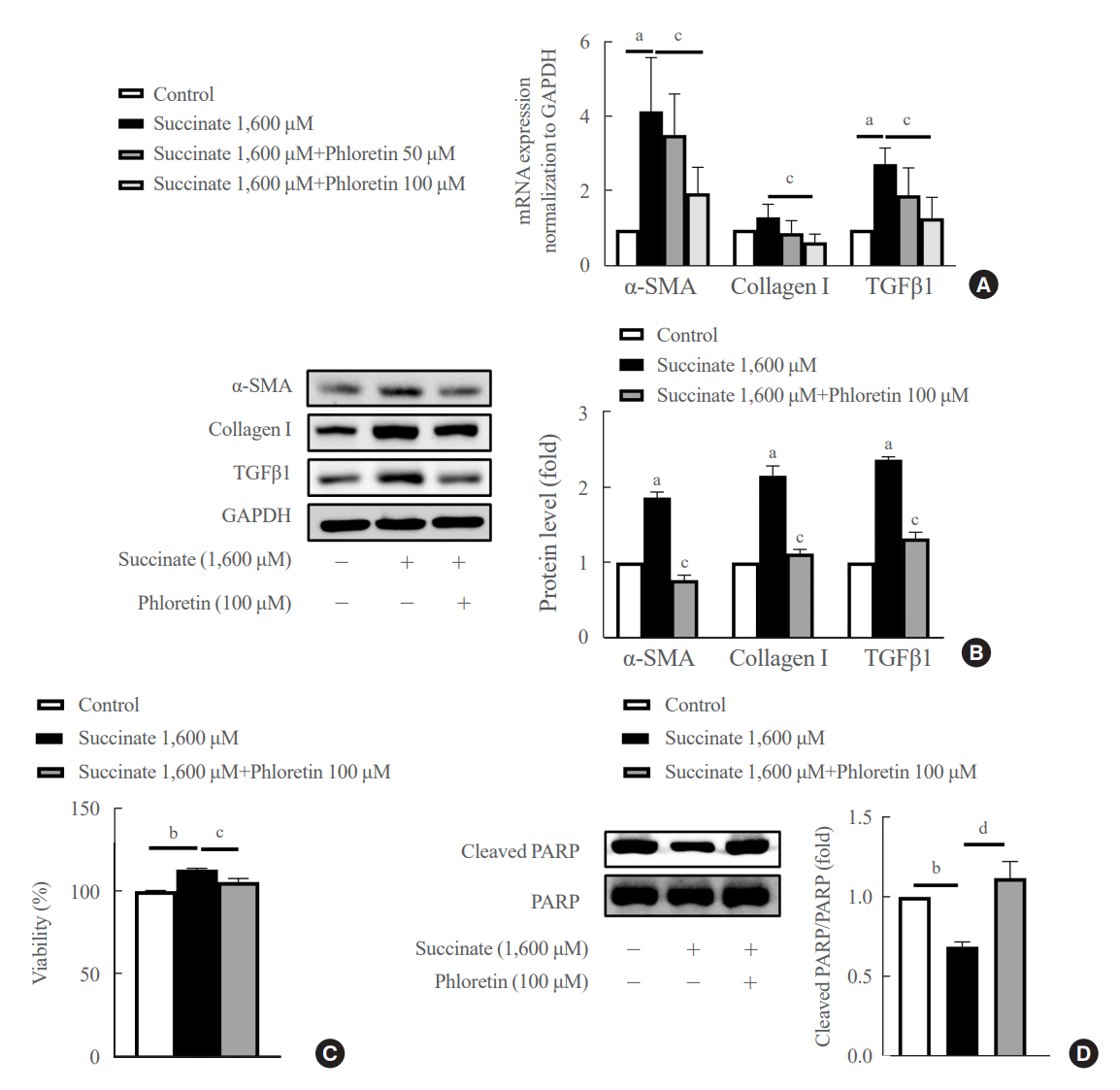
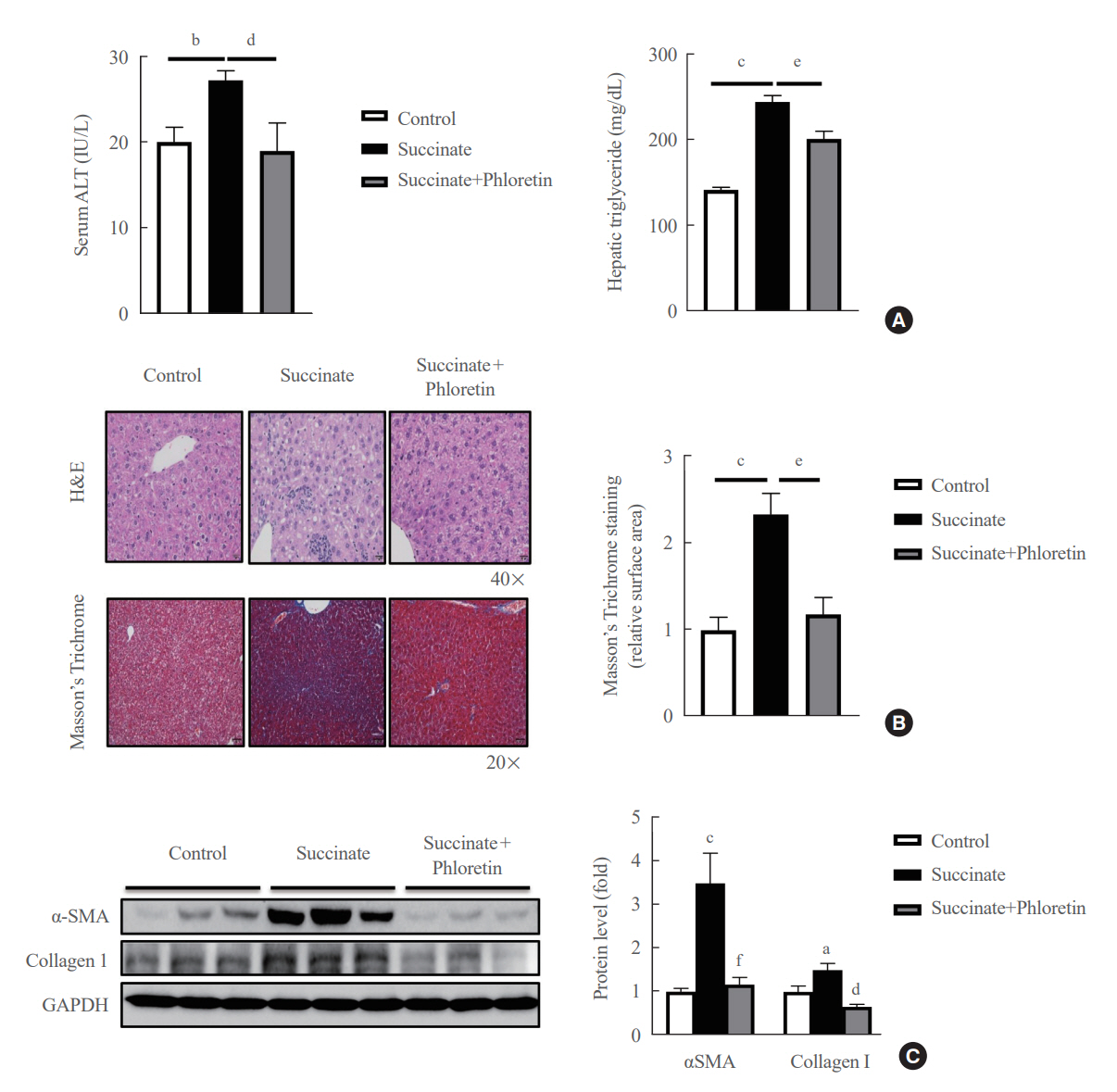
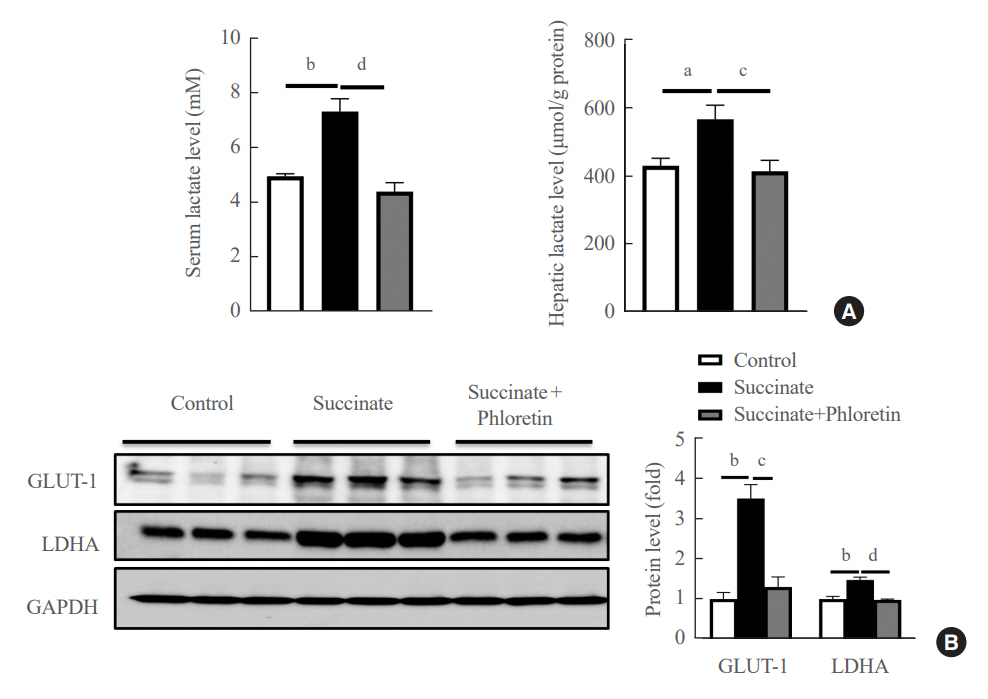
Phloretin can reduce the increase of fibrogenic markers and inhibits the proliferation, migration, and contraction caused by succinate in in vitro experiments. Moreover, an upregulation of proteins associated with aerobic glycolysis occurred during the activation of HSCs, which was attenuated by phloretin treatment. In in vivo experiments, intraperitoneal injection of phloretin decreased expression of fibrotic and glycolytic markers in the livers of mice with sodium succinate diet-induced liver fibrosis. These results suggest that aerobic glycolysis plays critical role in activation of HSCs and succinate can induce liver fibrosis in mice, whereas phloretin has therapeutic potential for treating hepatic fibrosis.
Conclusion
Intraperitoneal injection of phloretin attenuated succinate-induced hepatic fibrosis and alleviates the succinate-induced HSCs activation.
Figure
Reference
-
1. Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000; 275:2247–50.2. Eng FJ, Friedman SL. Fibrogenesis I: new insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000; 279:G7–11.3. Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001; 21:437–51.4. He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004; 429:188–93.5. Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest. 2008; 118:2526–34.6. Astiarraga B, Martinez L, Ceperuelo-Mallafre V, Llaurado G, Terron-Puig M, Rodriguez MM, et al. Impaired succinate response to a mixed meal in obesity and type 2 diabetes is normalized after metabolic surgery. Diabetes Care. 2020; 43:2581–7.7. Fernandez-Veledo S, Vendrell J. Gut microbiota-derived succinate: friend or foe in human metabolic diseases? Rev Endocr Metab Disord. 2019; 20:439–47.8. Ceperuelo-Mallafre V, Llaurado G, Keiran N, Benaiges E, Astiarraga B, Martinez L, et al. Preoperative circulating succinate levels as a biomarker for diabetes remission after bariatric surgery. Diabetes Care. 2019; 42:1956–65.9. van Diepen JA, Robben JH, Hooiveld GJ, Carmone C, Alsady M, Boutens L, et al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia. 2017; 60:1304–13.10. Li YH, Woo SH, Choi DH, Cho EH. Succinate causes α-SMA production through GPR91 activation in hepatic stellate cells. Biochem Biophys Res Commun. 2015; 463:853–8.11. Park SY, Le CT, Sung KY, Choi DH, Cho EH. Succinate induces hepatic fibrogenesis by promoting activation, proliferation, and migration, and inhibiting apoptosis of hepatic stellate cells. Biochem Biophys Res Commun. 2018; 496:673–8.12. Le CT, Nguyen G, Dong HN, Park SY, Cho YK, Choi DH, et al. Succinate induces liver damage and hepatic fibrosis in a mouse model. Keimyung Med J. 2022; 41:84–91.13. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009; 324:1029–33.14. Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001; 292:504–7.15. Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012; 143:1319–29.16. Rezk BM, Haenen GR, van der Vijgh WJ, Bast A. The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids. Biochem Biophys Res Commun. 2002; 295:9–13.17. Najafian M, Jahromi MZ, Nowroznejhad MJ, Khajeaian P, Kargar MM, Sadeghi M, et al. Phloridzin reduces blood glucose levels and improves lipids metabolism in streptozotocin-induced diabetic rats. Mol Biol Rep. 2012; 39:5299–306.18. Hassan M, El Yazidi C, Malezet-Desmoulins C, Amiot MJ, Margotat A. Gene expression profiling of 3T3-L1 adipocytes exposed to phloretin. J Nutr Biochem. 2010; 21:645–52.19. Shu G, Lu NS, Zhu XT, Xu Y, Du MQ, Xie QP, et al. Phloretin promotes adipocyte differentiation in vitro and improves glucose homeostasis in vivo. J Nutr Biochem. 2014; 25:1296–308.20. Chang WT, Huang WC, Liou CJ. Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chem. 2012; 134:972–9.21. Alsanea S, Gao M, Liu D. Phloretin prevents high-fat diet-induced obesity and improves metabolic homeostasis. AAPS J. 2017; 19:797–805.22. Lu Y, Chen J, Ren D, Yang X, Zhao Y. Hepatoprotective effects of phloretin against CCl4-induced liver injury in mice. Food Agric Immunol. 2017; 28:211–22.23. Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, et al. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009; 124:2210–9.24. Cho SJ, Moon JS, Lee CM, Choi AM, Stout-Delgado HW. Glucose transporter 1-dependent glycolysis is increased during aging-related lung fibrosis, and phloretin inhibits lung fibrosis. Am J Respir Cell Mol Biol. 2017; 56:521–31.25. Huang WC, Fang LW, Liou CJ. Phloretin attenuates allergic airway inflammation and oxidative stress in asthmatic mice. Front Immunol. 2017; 8:134.26. Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, et al. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014; 5:e1336.27. Mills EL, Pierce KA, Jedrychowski MP, Garrity R, Winther S, Vidoni S, et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018; 560:102–6.28. Lian N, Jin H, Zhang F, Wu L, Shao J, Lu Y, et al. Curcumin inhibits aerobic glycolysis in hepatic stellate cells associated with activation of adenosine monophosphate-activated protein kinase. IUBMB Life. 2016; 68:589–96.29. Gomes MT, Guimaraes ES, Marinho FV, Macedo I, Aguiar ER, Barber GN, et al. STING regulates metabolic reprogramming in macrophages via HIF-1α during Brucella infection. PLoS Pathog. 2021; 17:e1009597.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gemigliptin Alleviates Succinate-Induced Hepatic Stellate Cell Activation by Ameliorating Mitochondrial Dysfunction
- Role of cytoglobin, a novel radical scavenger, in stellate cell activation and hepatic fibrosis
- Succinate Induces Liver Damage and Hepatic Fibrosis in a Mouse Model
- Reversal of liver cirrhosis: current evidence and expectations
- The Role of Activated Hepatic Stellate Cells in Liver Fibrosis, Portal Hypertension and Cancer Angiogenesis