Diabetes Metab J.
2023 Jul;47(4):454-469. 10.4093/dmj.2022.0442.
Rediscovering Primary Cilia in Pancreatic Islets
- Affiliations
-
- 1Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2544725
- DOI: http://doi.org/10.4093/dmj.2022.0442
Abstract
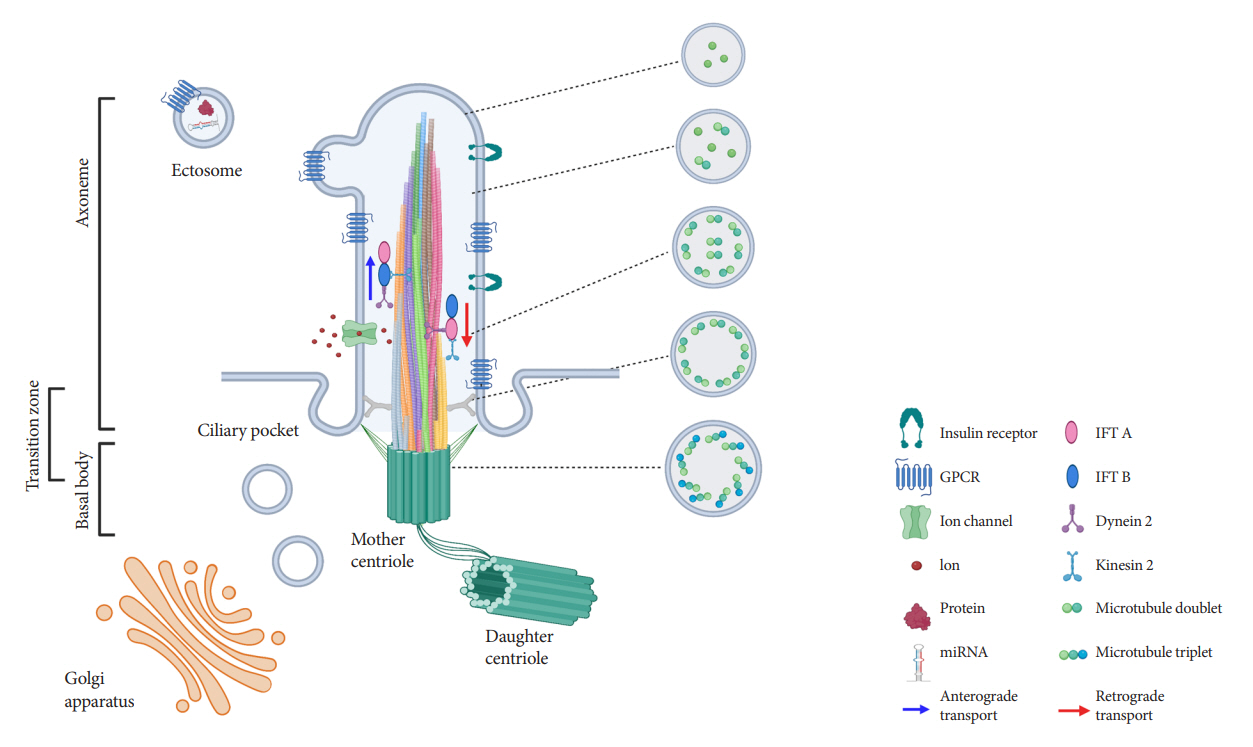
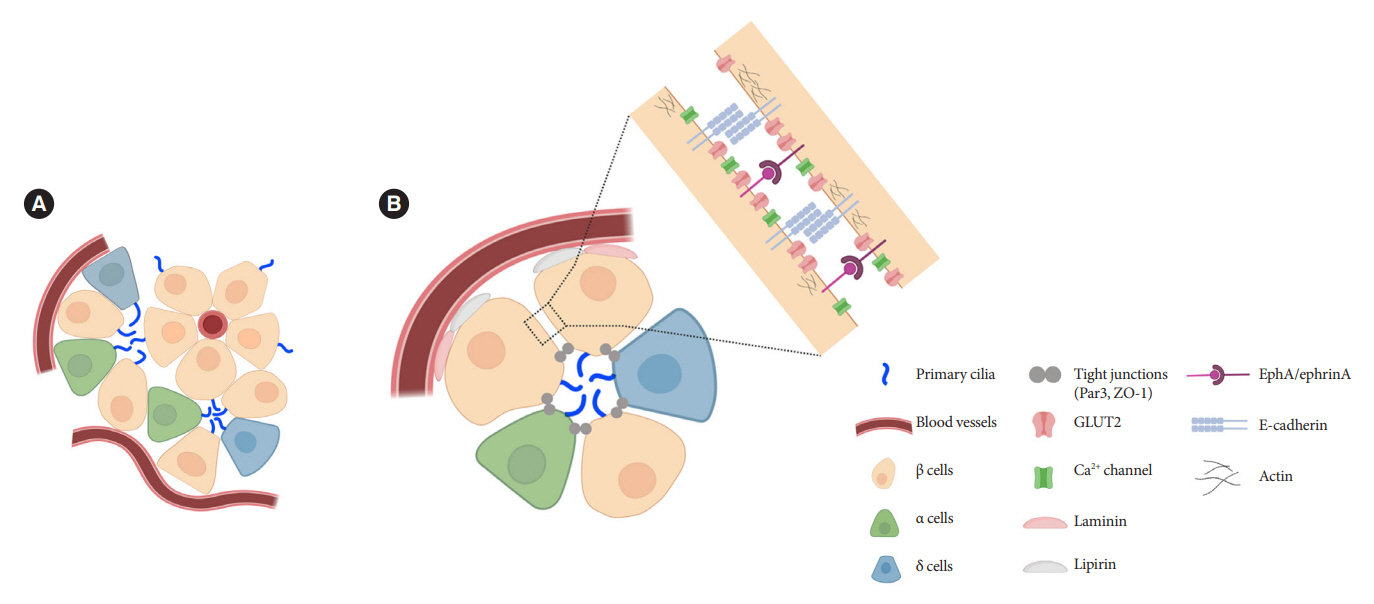
- Primary cilia are microtubule-based sensory and signaling organelles on the surfaces of most eukaryotic cells. Despite their early description by microscopy studies, islet cilia had not been examined in the functional context until recent decades. In pancreatic islets as in other tissues, primary cilia facilitate crucial developmental and signaling pathways in response to extracellular stimuli. Many human developmental and genetic disorders are associated with ciliary dysfunction, some manifesting as obesity and diabetes. Understanding the basis for metabolic diseases in human ciliopathies has been aided by close examination of cilia action in pancreatic islets at cellular and molecular levels. In this article, we review the evidence for ciliary expression on islet cells, known roles of cilia in pancreas development and islet hormone secretion, and summarize metabolic manifestations of human ciliopathy syndromes. We discuss emerging data on primary cilia regulation of islet cell signaling and the structural basis of cilia-mediated cell crosstalk, and offer our interpretation on the role of cilia in glucose homeostasis and human diseases.
Figure
Reference
-
1. Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007; 8:880–93.
Article2. Van Leeuwenhoek A. Concerning little animals by him observed in rain-well-sea- and snow water: as also in water wherein pepper had lain infused. Phil Trans R Soc. 1677; 12:821–31.3. Zimmermann K. Beitrage zur Kenntnis einiger Drasen und Epithelien. Arch Mikrosk Anat. 1898; 52:552–706.4. Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976; 193:317–9.
Article5. Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000; 151:709–18.6. Pala R, Alomari N, Nauli SM. Primary cilium-dependent signaling mechanisms. Int J Mol Sci. 2017; 18:2272.
Article7. Nishimura Y, Kasahara K, Shiromizu T, Watanabe M, Inagaki M. Primary cilia as signaling hubs in health and disease. Adv Sci (Weinh). 2018; 6:1801138.
Article8. Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST. Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol. 2019; 15:199–219.
Article9. Wu CT, Hilgendorf KI, Bevacqua RJ, Hang Y, Demeter J, Kim SK, et al. Discovery of ciliary G protein-coupled receptors regulating pancreatic islet insulin and glucagon secretion. Genes Dev. 2021; 35:1243–55.
Article10. Yang DJ, Hong J, Kim KW. Hypothalamic primary cilium: a hub for metabolic homeostasis. Exp Mol Med. 2021; 53:1109–15.
Article11. Brewer KM, Brewer KK, Richardson NC, Berbari NF. Neuronal cilia in energy homeostasis. Front Cell Dev Biol. 2022; 10:1082141.
Article12. Sun JS, Yang DJ, Kinyua AW, Yoon SG, Seong JK, Kim J, et al. Ventromedial hypothalamic primary cilia control energy and skeletal homeostasis. J Clin Invest. 2021; 131:e138107.
Article13. Bush A, Chodhari R, Collins N, Copeland F, Hall P, Harcourt J, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007; 92:1136–40.
Article14. Mujahid S, Hunt KF, Cheah YS, Forsythe E, Hazlehurst JM, Sparks K, et al. The endocrine and metabolic characteristics of a large Bardet-Biedl syndrome clinic population. J Clin Endocrinol Metab. 2018; 103:1834–41.
Article15. Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, et al. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alstrom syndrome. Nat Genet. 2002; 31:74–8.
Article16. Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004; 131:3457–67.17. Barker AR, Thomas R, Dawe HR. Meckel-Gruber syndrome and the role of primary cilia in kidney, skeleton, and central nervous system development. Organogenesis. 2014; 10:96–107.
Article18. Fleming LR, Doherty DA, Parisi MA, Glass IA, Bryant J, Fischer R, et al. Prospective evaluation of kidney disease in Joubert syndrome. Clin J Am Soc Nephrol. 2017; 12:1962–73.
Article19. Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003; 425:628–33.
Article20. Engle SE, Bansal R, Antonellis PJ, Berbari NF. Cilia signaling and obesity. Semin Cell Dev Biol. 2021; 110:43–50.
Article21. Lee CH, Kang GM, Kim MS. Mechanisms of weight control by primary cilia. Mol Cells. 2022; 45:169–76.
Article22. Yang DJ, Tran LT, Yoon SG, Seong JK, Shin DM, Choi YH, et al. Primary cilia regulate adaptive responses to fasting. Metabolism. 2022; 135:155273.
Article23. Pollara L, Sottile V, Valente EM. Patient-derived cellular models of primary ciliopathies. J Med Genet. 2022; 59:517–27.
Article24. Sun S, Fisher RL, Bowser SS, Pentecost BT, Sui H. Three-dimensional architecture of epithelial primary cilia. Proc Natl Acad Sci U S A. 2019; 116:9370–9.
Article25. Kiesel P, Alvarez Viar G, Tsoy N, Maraspini R, Gorilak P, Varga V, et al. The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nat Struct Mol Biol. 2020; 27:1115–24.
Article26. Xu CS, Pang S, Shtengel G, Muller A, Ritter AT, Hoffman HK, et al. An open-access volume electron microscopy atlas of whole cells and tissues. Nature. 2021; 599:147–51.
Article27. Green WR. Abnormal cilia in human pancreas. Hum Pathol. 1980; 11:686–7.
Article28. Bockman DE, Buchler M, Beger HG. Structure and function of specialized cilia in the exocrine pancreas. Int J Pancreatol. 1986; 1:21–8.
Article29. Aughsteen AA. The ultrastructure of primary cilia in the endocrine and excretory duct cells of the pancreas of mice and rats. Eur J Morphol. 2001; 39:277–83.
Article30. Nachury MV, Mick DU. Establishing and regulating the composition of cilia for signal transduction. Nat Rev Mol Cell Biol. 2019; 20:389–405.
Article31. Wang L, Dynlacht BD. The regulation of cilium assembly and disassembly in development and disease. Development. 2018; 145:dev151407.
Article32. Heydeck W, Fievet L, Davis EE, Katsanis N. The complexity of the cilium: spatiotemporal diversity of an ancient organelle. Curr Opin Cell Biol. 2018; 55:139–49.
Article33. Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009; 20:278–88.
Article34. Nager AR, Goldstein JS, Herranz-Perez V, Portran D, Ye F, Garcia-Verdugo JM, et al. An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling. Cell. 2017; 168:252–63.
Article35. Wood CR, Rosenbaum JL. Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol. 2015; 25:276–85.
Article36. Volz AK, Frei A, Kretschmer V, de Jesus Domingues AM, Ketting RF, Ueffing M, et al. Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nat Commun. 2021; 12:5671.
Article37. Wang J, Nikonorova IA, Silva M, Walsh JD, Tilton PE, Gu A, et al. Sensory cilia act as a specialized venue for regulated extracellular vesicle biogenesis and signaling. Curr Biol. 2021; 31:3943–51.
Article38. Lacy PE. Electron microscopy of the normal islets of Langerhans: studies in the dog, rabbit, guinea pig and rat. Diabetes. 1957; 6:498–507.
Article39. Munger BL. A light and electron microscopic study of cellular differentiation in the pancreatic islets of the mouse. Am J Anat. 1958; 103:275–311.
Article40. Ait-Lounis A, Baas D, Barras E, Benadiba C, Charollais A, Nlend Nlend R, et al. Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes. 2007; 56:950–9.
Article41. Zhang Q, Davenport JR, Croyle MJ, Haycraft CJ, Yoder BK. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Lab Invest. 2005; 85:45–64.
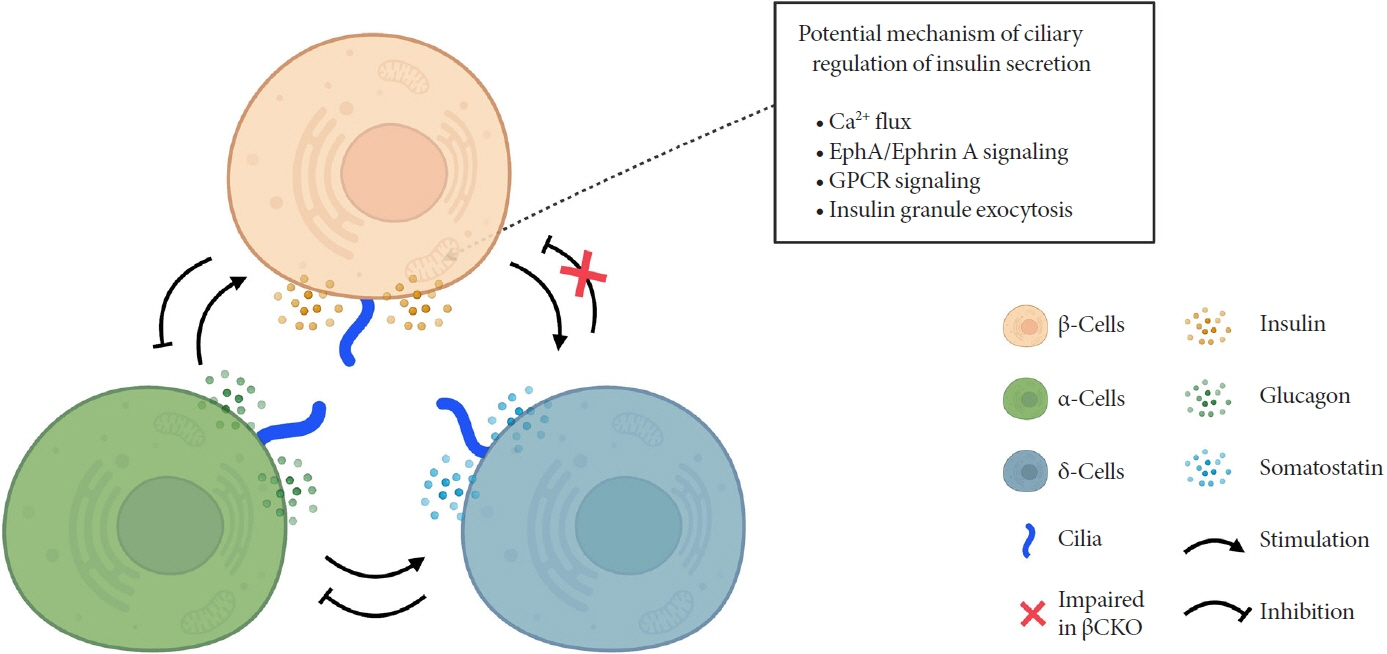
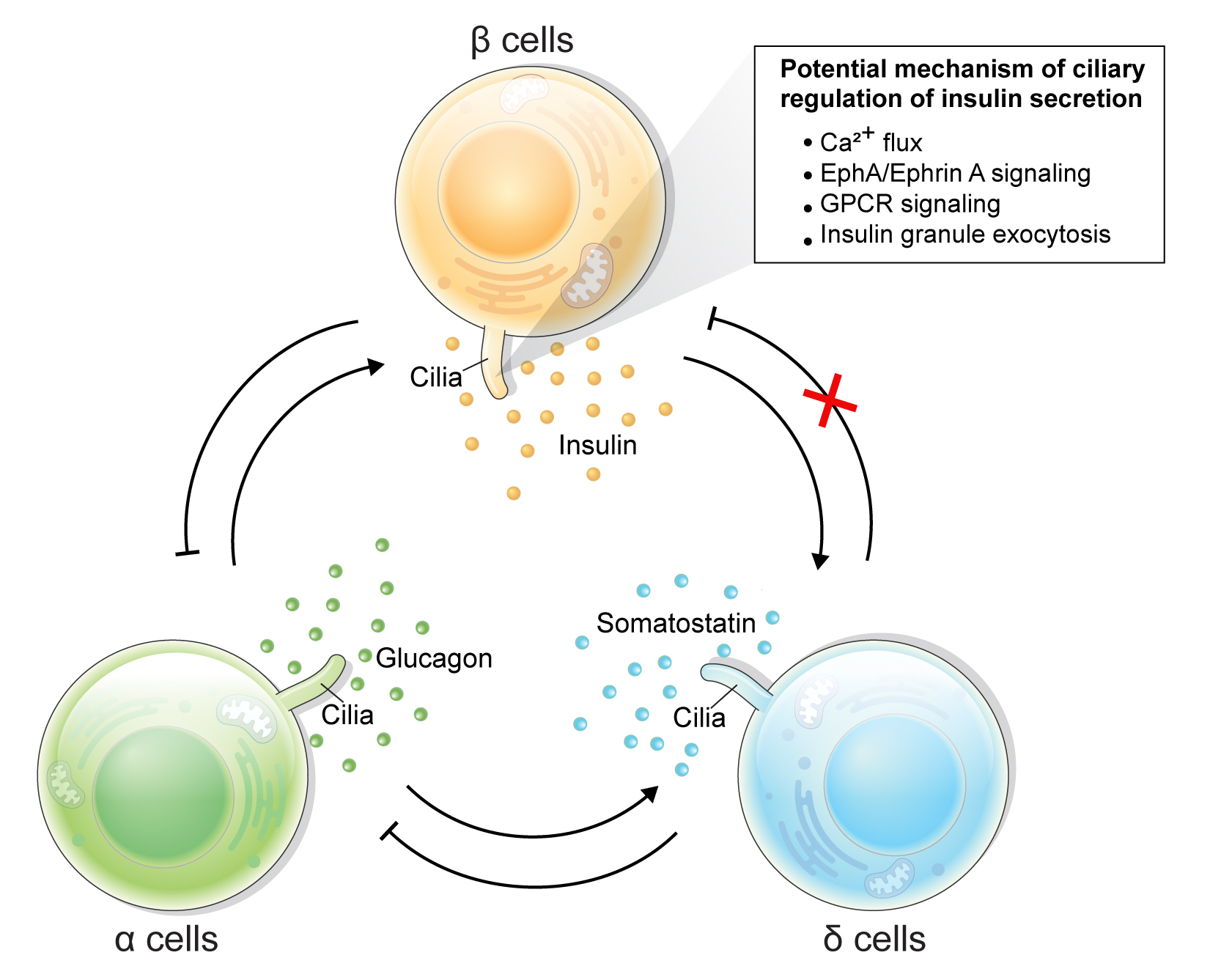
Article42. Hughes JW, Cho JH, Conway HE, DiGruccio MR, Ng XW, Roseman HF, et al. Primary cilia control glucose homeostasis via islet paracrine interactions. Proc Natl Acad Sci U S A. 2020; 117:8912–23.
Article43. Cyge B, Voronina V, Hoque M, Kim EN, Hall J, Bailey-Lundberg JM, et al. Loss of the ciliary protein Chibby1 in mice leads to exocrine pancreatic degeneration and pancreatitis. Sci Rep. 2021; 11:17220.
Article44. Phelps EA, Cianciaruso C, Santo-Domingo J, Pasquier M, Galliverti G, Piemonti L, et al. Advances in pancreatic islet monolayer culture on glass surfaces enable super-resolution microscopy and insights into beta cell ciliogenesis and proliferation. Sci Rep. 2017; 7:45961.
Article45. Greider MH, Elliott DW. Electron microscopy of human pancreatic tumors of islet cell origin. Am J Pathol. 1964; 44:663–78.46. Hendley AM, Rao AA, Leonhardt L, Ashe S, Smith JA, Giacometti S, et al. Single-cell transcriptome analysis defines heterogeneity of the murine pancreatic ductal tree. Elife. 2021; 10:e67776.
Article47. Kluth O, Stadion M, Gottmann P, Aga H, Jahnert M, Scherneck S, et al. Decreased expression of cilia genes in pancreatic islets as a risk factor for type 2 diabetes in mice and humans. Cell Rep. 2019; 26:3027–36.
Article48. Walker JT, Saunders DC, Rai V, Dai C, Orchard P, Hopkirk AL, et al. RFX6-mediated dysregulation defines human β cell dysfunction in early type 2 diabetes. bioRxiv. 2021; Dec. 17. [Preprint]. https://doi.org/10.1101/2021.12.16.466282.
Article49. Cho JH, Li ZA, Zhu L, Muegge BD, Roseman HF, Lee EY, et al. Islet primary cilia motility controls insulin secretion. Sci Adv. 2022; 8:eabq8486.
Article50. Orci L, Thorens B, Ravazzola M, Lodish HF. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989; 245:295–7.
Article51. Tomita T. Immunocytochemical localization of glucose transporter-2 (GLUT-2) in pancreatic islets and islet cell tumors. Endocr Pathol. 1999; 10:213–21.
Article52. Low JT, Zavortink M, Mitchell JM, Gan WJ, Do OH, Schwiening CJ, et al. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia. 2014; 57:1655–63.
Article53. Yamamoto M, Kataoka K. A comparative study on the intercellular canalicular system and intercellular junctions in the pancreatic islets of some rodents. Arch Histol Jpn. 1984; 47:485–93.
Article54. Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, et al. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab. 2009; 10:296–308.55. Gan WJ, Zavortink M, Ludick C, Templin R, Webb R, Webb R, et al. Cell polarity defines three distinct domains in pancreatic β-cells. J Cell Sci. 2017; 130:143–51.56. Cottle L, Gan WJ, Gilroy I, Samra JS, Gill AJ, Loudovaris T, et al. Structural and functional polarisation of human pancreatic beta cells in islets from organ donors with and without type 2 diabetes. Diabetologia. 2021; 64:618–29.
Article57. Geron E, Boura-Halfon S, Schejter ED, Shilo BZ. The edges of pancreatic islet β cells constitute adhesive and signaling microdomains. Cell Rep. 2015; 10:317–25.
Article58. Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001; 184:71–9.
Article59. Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003; 33:129–37.
Article60. Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008; 117:1161–71.
Article61. Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998; 95:829–37.
Article62. Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J Cell Biol. 1999; 145:825–36.
Article63. Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005; 437:1018–21.
Article64. Katoh TA, Omori T, Mizuno K, Sai X, Minegishi K, Ikawa Y, et al. Immotile cilia mechanically sense the direction of fluid flow for left-right determination. Science. 2023; 379:66–71.
Article65. Djenoune L, Mahamdeh M, Truong TV, Nguyen CT, Fraser SE, Brueckner M, et al. Cilia function as calcium-mediated mechanosensors that instruct left-right asymmetry. Science. 2023; 379:71–8.
Article66. Sobkowicz HM, Slapnick SM, August BK. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol. 1995; 24:633–53.
Article67. Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997; 272(1 Pt 2):F132–8.
Article68. Battle C, Ott CM, Burnette DT, Lippincott-Schwartz J, Schmidt CF. Intracellular and extracellular forces drive primary cilia movement. Proc Natl Acad Sci U S A. 2015; 112:1410–5.
Article69. Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, et al. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011; 13:351–60.
Article70. Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, et al. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol. 2011; 13:402–11.
Article71. Jackson PK. Do cilia put brakes on the cell cycle? Nat Cell Biol. 2011; 13:340–2.
Article72. Cano DA, Sekine S, Hebrok M. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology. 2006; 131:1856–69.
Article73. Gallagher AR, Esquivel EL, Briere TS, Tian X, Mitobe M, Menezes LF, et al. Biliary and pancreatic dysgenesis in mice harboring a mutation in Pkhd1. Am J Pathol. 2008; 172:417–29.
Article74. Collin GB, Cyr E, Bronson R, Marshall JD, Gifford EJ, Hicks W, et al. Alms1-disrupted mice recapitulate human Alstrom syndrome. Hum Mol Genet. 2005; 14:2323–33.75. Lu W, Peissel B, Babakhanlou H, Pavlova A, Geng L, Fan X, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997; 17:179–81.
Article76. Morgan D, Turnpenny L, Goodship J, Dai W, Majumder K, Matthews L, et al. Inversin, PCNTft-right axis pathway, is partially deleted in the inv mouse. Nat Genet. 1998; 20:149–56.
Article77. Pierreux CE, Poll AV, Kemp CR, Clotman F, Maestro MA, Cordi S, et al. The transcription factor hepatocyte nuclear factor-6 controls the development of pancreatic ducts in the mouse. Gastroenterology. 2006; 130:532–41.
Article78. Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, et al. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int. 2007; 72:328–36.
Article79. Volta F, Scerbo MJ, Seelig A, Wagner R, O’Brien N, Gerst F, et al. Glucose homeostasis is regulated by pancreatic β-cell cilia via endosomal EphA-processing. Nat Commun. 2019; 10:5686.
Article80. Gerdes JM, Christou-Savina S, Xiong Y, Moede T, Moruzzi N, Karlsson-Edlund P, et al. Ciliary dysfunction impairs beta-cell insulin secretion and promotes development of type 2 diabetes in rodents. Nat Commun. 2014; 5:5308.
Article81. Arsov T, Silva DG, O’Bryan MK, Sainsbury A, Lee NJ, Kennedy C, et al. Fat Aussie: a new Alstrom syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol Endocrinol. 2006; 20:1610–22.82. Cervantes S, Lau J, Cano DA, Borromeo-Austin C, Hebrok M. Primary cilia regulate Gli/Hedgehog activation in pancreas. Proc Natl Acad Sci U S A. 2010; 107:10109–14.
Article83. Landsman L, Parent A, Hebrok M. Elevated Hedgehog/Gli signaling causes beta-cell dedifferentiation in mice. Proc Natl Acad Sci U S A. 2011; 108:17010–5.84. Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes. 2012; 61:1700–7.
Article85. Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, et al. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007; 129:359–70.
Article86. Kalwat MA, Thurmond DC. Signaling mechanisms of glucose-induced F-actin remodeling in pancreatic islet β cells. Exp Mol Med. 2013; 45:e37.
Article87. Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006; 103:2334–9.
Article88. Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005; 53:1087–97.
Article89. Nechipurenko IV. The enigmatic role of lipids in cilia signaling. Front Cell Dev Biol. 2020; 8:777.
Article90. Conduit SE, Vanhaesebroeck B. Phosphoinositide lipids in primary cilia biology. Biochem J. 2020; 477:3541–65.
Article91. Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S. Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol. 2017; 216:743–60.
Article92. Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G proteincoupled receptors into primary cilia. Genes Dev. 2010; 24:2180–93.
Article93. Dyson JM, Conduit SE, Feeney SJ, Hakim S, DiTommaso T, Fulcher AJ, et al. INPP5E regulates phosphoinositide-dependent cilia transition zone function. J Cell Biol. 2017; 216:247–63.
Article94. Conduit SE, Ramaswamy V, Remke M, Watkins DN, Wainwright BJ, Taylor MD, et al. A compartmentalized phosphoinositide signaling axis at cilia is regulated by INPP5E to maintain cilia and promote sonic Hedgehog medulloblastoma. Oncogene. 2017; 36:5969–84.
Article95. Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012; 13:608–18.
Article96. Mykytyn K, Askwith C. G-protein-coupled receptor signaling in cilia. Cold Spring Harb Perspect Biol. 2017; 9:a028183.
Article97. Schou KB, Pedersen LB, Christensen ST. Ins and outs of GPCR signaling in primary cilia. EMBO Rep. 2015; 16:1099–113.98. Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017; 16:829–42.
Article99. Hilgendorf KI, Johnson CT, Jackson PK. The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr Opin Cell Biol. 2016; 39:84–92.
Article100. Truong ME, Bilekova S, Choksi SP, Li W, Bugaj LJ, Xu K, et al. Vertebrate cells differentially interpret ciliary and extraciliary cAMP. Cell. 2021; 184:2911–26.
Article101. Sanchez GM, Incedal TC, Prada J, O’Callaghan P, Dyachok O, Echeverry S, et al. The β-cell primary cilium is an autonomous Ca2+ compartment for paracrine GABA signaling. J Cell Biol. 2023; 222:e202108101.
Article102. Iwanaga T, Miki T, Takahashi-Iwanaga H. Restricted expression of somatostatin receptor 3 to primary cilia in the pancreatic islets and adenohypophysis of mice. Biomed Res. 2011; 32:73–81.
Article103. O’Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, et al. An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia. 2013; 2:8.
Article104. Li ZA, Cho JH, Woodhams LG, Hughes JW. Fluorescence imaging of beta cell primary cilia. Front Endocrinol (Lausanne). 2022; 13:1004136.
Article105. Girard D, Petrovsky N. Alstrom syndrome: insights into the pathogenesis of metabolic disorders. Nat Rev Endocrinol. 2011; 7:77–88.
Article106. Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, et al. New Alstrom syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005; 165:675–83.
Article107. Pietrzak-Nowacka M, Safranow K, Byra E, Nowosiad M, Marchelek-Mysliwiec M, Ciechanowski K. Glucose metabolism parameters during an oral glucose tolerance test in patients with autosomal dominant polycystic kidney disease. Scand J Clin Lab Invest. 2010; 70:561–7.
Article108. Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science. 2008; 319:816–9.
Article109. Huang-Doran I, Bicknell LS, Finucane FM, Rocha N, Porter KM, Tung YC, et al. Genetic defects in human pericentrin are associated with severe insulin resistance and diabetes. Diabetes. 2011; 60:925–35.
Article110. Lee SH, Somlo S. Cyst growth, polycystins, and primary cilia in autosomal dominant polycystic kidney disease. Kidney Res Clin Pract. 2014; 33:73–8.
Article111. Kathem SH, Mohieldin AM, Nauli SM. The roles of primary cilia in polycystic kidney disease. AIMS Mol Sci. 2014; 1:27–46.
Article112. Walker RV, Keynton JL, Grimes DT, Sreekumar V, Williams DJ, Esapa C, et al. Ciliary exclusion of polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model. Nat Commun. 2019; 10:4072.
Article113. Marshall JD, Maffei P, Collin GB, Naggert JK. Alstrom syndrome: genetics and clinical overview. Curr Genomics. 2011; 12:225–35.
Article114. Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al. Gene Reviews. Seattle: University of Washington, Seattle;1993-2023. Chapter, Bardet-Biedl syndrome overview [cited 2023 Apr 6]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1363.115. Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013; 21:8–13.
Article116. Suspitsin EN, Imyanitov EN. Bardet-Biedl syndrome. Mol Syndromol. 2016; 7:62–71.
Article117. Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008; 82:959–70.118. Frank V, Habbig S, Bartram MP, Eisenberger T, Veenstra-Knol HE, Decker C, et al. Mutations in NEK8 link multiple organ dysplasia with altered Hippo signalling and increased c-MYC expression. Hum Mol Genet. 2013; 22:2177–85.
Article119. Franco B, Thauvin-Robinet C. Update on oral-facial-digital syndromes (OFDS). Cilia. 2016; 5:12.
Article120. Tirosh A, Sadowski SM, Linehan WM, Libutti SK, Patel D, Nilubol N, et al. Association of VHL genotype with pancreatic neuroendocrine tumor phenotype in patients with von Hippel-Lindau disease. JAMA Oncol. 2018; 4:124–6.
Article121. van Asselt SJ, de Vries EG, van Dullemen HM, Brouwers AH, Walenkamp AM, Giles RH, et al. Pancreatic cyst development: insights from von Hippel-Lindau disease. Cilia. 2013; 2:3.
Article122. Jurczyk A, Gromley A, Redick S, San Agustin J, Witman G, Pazour GJ, et al. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol. 2004; 166:637–43.
Article123. Censin JC, Nowak C, Cooper N, Bergsten P, Todd JA, Fall T. Childhood adiposity and risk of type 1 diabetes: a Mendelian randomization study. PLoS Med. 2017; 14:e1002362.
Article124. Grarup N, Moltke I, Andersen MK, Dalby M, Vitting-Seerup K, Kern T, et al. Loss-of-function variants in ADCY3 increase risk of obesity and type 2 diabetes. Nat Genet. 2018; 50:172–4.
Article125. Saxena R, Hivert MF, Langenberg C, Tanaka T, Pankow JS, Vollenweider P, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010; 42:142–8.126. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010; 42:105–16.127. Chabosseau P, Yong F, Delgadillo-Silva LF, Lee EY, Melhem R, Li S, et al. Molecular phenotyping of single pancreatic islet leader beta cells by “Flash-Seq”. Life Sci. 2023; 316:121436.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interplay Between Primary Cilia and Autophagy and Its Controversial Roles in Cancer
- Can Tissue Cilia Lengths and Urine Cilia Proteins Be Markers of Kidney Diseases?
- Effects of Free Fatty Acid on Insulin Secretion in Cultured Rat Pancreatic Islets
- Comparative study of endocrine cells in the principal pancreatic islets of two teleosts, Silurus asotus (Siluridae) and Siniperca scherzeri (Centropomidae)
- Immunocytochemical Expression of Amylin in Pancreatic Islets of Man, Rabbit and Guinea Pig