Ann Surg Treat Res.
2023 Jul;105(1):10-19. 10.4174/astr.2023.105.1.10.
Trends of axillary surgery in breast cancer patients with axillary lymph node metastasis: a comprehensive single-center retrospective study
- Affiliations
-
- 1Breast Division, Department of Surgery, Myongji Hospital, Hanyang University School of Medicine, Goyang, Korea
- 2Department of Surgery, Hyundae Hospital in educational cooperation with Chung-Ang University Medical System, Namyangju, Korea
- 3Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2544598
- DOI: http://doi.org/10.4174/astr.2023.105.1.10
Abstract
- Purpose
Based on the results of previous trials, de-escalation of axillary surgery after neoadjuvant chemotherapy (NAC) has increased in patients with axillary lymph node (ALN) metastasis at presentation. This study aimed to review the trends of axillary surgery by time period and molecular subtype in patients with ALN metastasis.
Methods
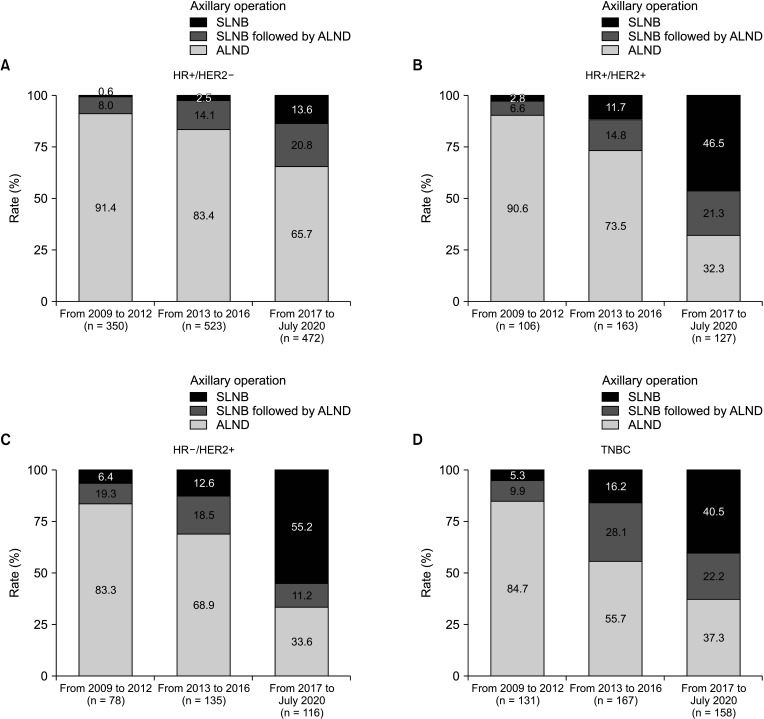
We analyzed the rates of sentinel lymph node biopsy (SLNB) and ALN dissection (ALND) based on time period and subtype. The time period was divided into 3 subperiods to determine the rate of axillary surgery type over time (period 1, from 2009 to 2012; period 2, from 2013 to 2016; and period 3, from 2017 to July 2019).
Results
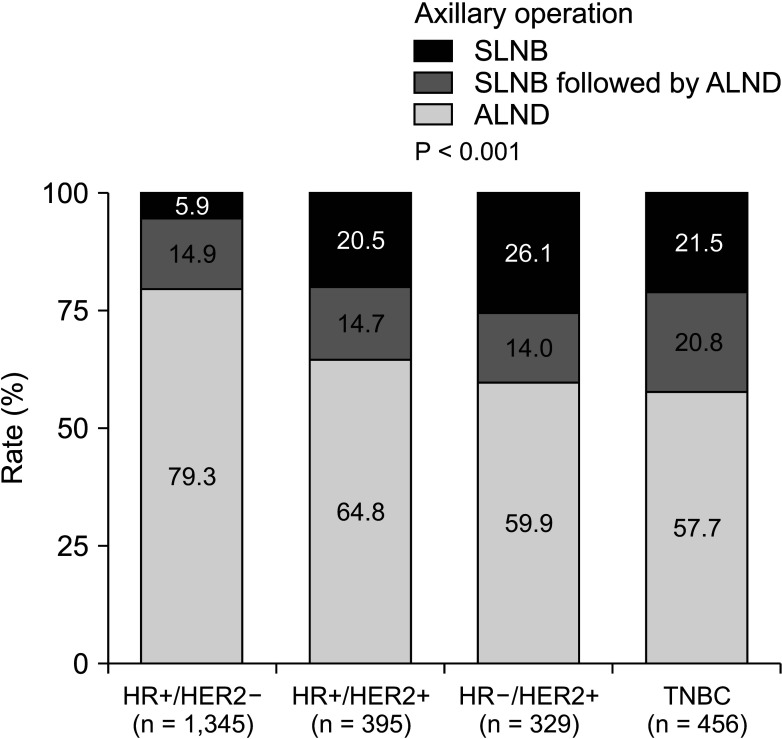
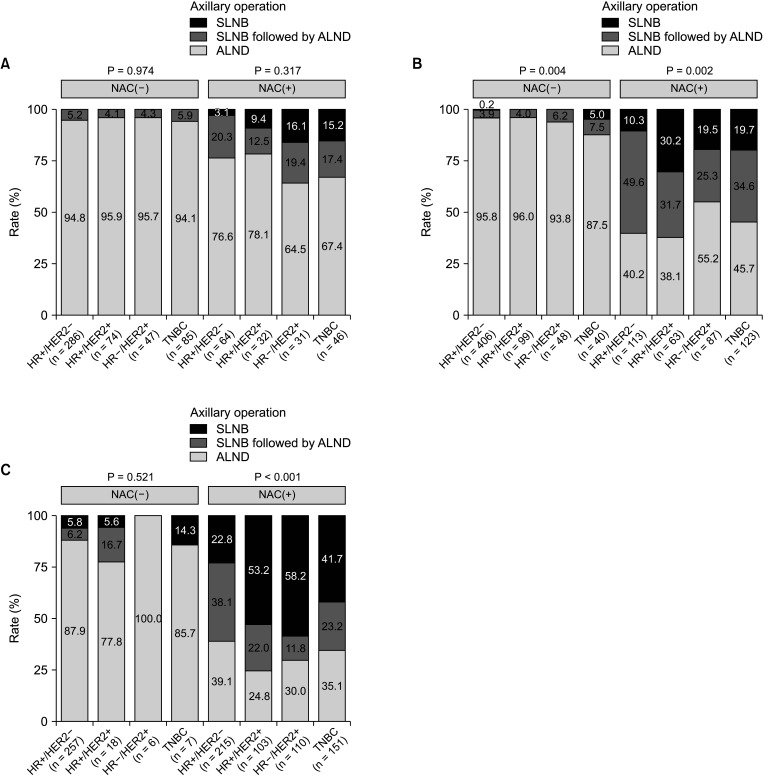
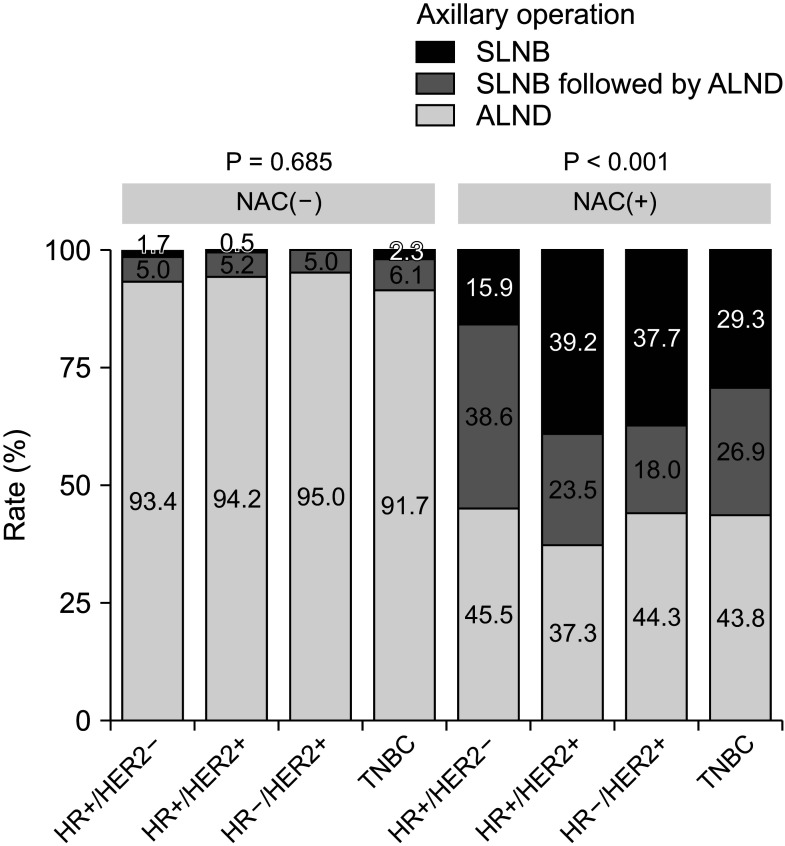
From 2009 to July 2019, 2,525 breast cancer patients underwent surgery. Based on subtype, the ALND rate of hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2–) disease decreased by 13.0% from period 1 to period 3 (period 1, 99.4%; period 2, 97.5%; and period 3, 86.4%; P < 0.001). Conversely, the ALND rate in HR+/HER2+, HR–/HER2+, and triple-negative breast cancer (TNBC) significantly decreased by 43.7%, 48.8%, and 35.2% in period 1, period 2, and period 3, respectively (P < 0.001). In the patient group receiving NAC, HR+/HER2– had a significantly higher ALND rate (84.1%) than HR+/HER2+, HR–/HER2+, and TNBC (60.8%, 62.3%, and 70.7%, respectively; P < 0.001).
Conclusion
The SLNB rate in patients with ALN metastasis has increased over time. However, the ALND rate in HR+/ HER2– was significantly higher than in other subtypes.
Keyword
Figure
Reference
-
1. Tadros AB, Yang WT, Krishnamurthy S, Rauch GM, Smith BD, Valero V, et al. Identification of patients with documented pathologic complete response in the breast after neoadjuvant chemotherapy for omission of axillary surgery. JAMA Surg. 2017; 152:665–670. PMID: 28423171.2. Wang Y, Li L, Liu X, Wang Y, Tang Z, Wu Y, et al. Treatment response correlation between primary tumor and axillary lymph nodes after neoadjuvant therapy in breast cancer: a retrospective study based on real-world data. Gland Surg. 2021; 10:656–669. PMID: 33708548.3. Corsi F, Albasini S, Sorrentino L, Armatura G, Carolla C, Chiappa C, et al. Development of a novel nomogram-based online tool to predict axillary status after neoadjuvant chemotherapy in cN+ breast cancer: a multicentre study on 1,950 patients. Breast. 2021; 60:131–137. PMID: 34624755.4. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013; 14:609–618. PMID: 23683750.5. Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015; 33:258–264. PMID: 25452445.6. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013; 310:1455–1461. PMID: 24101169.7. Weiss A, Campbell J, Ballman KV, Sikov WM, Carey LA, Hwang ES, et al. Factors associated with nodal pathologic complete response among breast cancer patients treated with neoadjuvant chemotherapy: results of CALGB 40601 (HER2+) and 40603 (Triple-Negative) (Alliance). Ann Surg Oncol. 2021; 28:5960–5971. PMID: 33821344.8. Samiei S, Simons JM, Engelen SM, Beets-Tan RG, Classe JM, Smidt ML, et al. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg. 2021; 156:e210891. PMID: 33881478.9. Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014; 260:608–616. PMID: 25203877.10. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172. PMID: 24529560.11. von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014; 15:747–756. PMID: 24794243.12. Kim JY, Nam SJ, Lee JE, Yu J, Chae BJ, Lee SK, et al. Real world evidence of neoadjuvant docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP) in patients with HER2-positive early or locally advanced breast cancer: a single-institutional clinical experience. Cancer Res Treat. 2022; 54:1091–1098. PMID: 35008143.13. Tee SR, Devane LA, Evoy D, Rothwell J, Geraghty J, Prichard RS, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018; 105:1541–1552. PMID: 30311642.14. Myers SP, Ahrendt GM, Lee JS, Steiman JG, Soran A, Johnson RR, et al. Association of tumor molecular subtype and stage with breast and axillary pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2021; 28:8636–8642. PMID: 34142288.15. Laot L, Laas E, Girard N, Dumas E, Daoud E, Grandal B, et al. The prognostic value of lymph node involvement after neoadjuvant chemotherapy is different among breast cancer subtypes. Cancers (Basel). 2021; 13:171. PMID: 33418983.16. Kim JY, Park HS, Kim S, Ryu J, Park S, Kim SI. Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Medicine (Baltimore). 2015; 94:e1720. PMID: 26512562.17. Enokido K, Watanabe C, Nakamura S, Ogiya A, Osako T, Akiyama F, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer. Clin Breast Cancer. 2016; 16:299–304. PMID: 26993216.18. Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016; 375:717–729. PMID: 27557300.19. Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021; 385:2336–2347. PMID: 34914339.20. Silva LR, Vargas RF, Shinzato JY, Derchain SF, Ramalho S, Zeferino LC. Association of menopausal status, expression of progesterone receptor and Ki67 to the clinical response to neoadjuvant chemotherapy in luminal breast cancer. Rev Bras Ginecol Obstet. 2019; 41:710–717. PMID: 31856290.21. Pease AM, Riba LA, Gruner RA, Tung NM, James TA. Oncotype DX® recurrence score as a predictor of response to neoadjuvant chemotherapy. Ann Surg Oncol. 2019; 26:366–371. PMID: 30542840.22. Pardo JA, Fan B, Mele A, Serres S, Valero MG, Emhoff I, et al. The role of oncotype DX® recurrence score in predicting axillary response after neoadjuvant chemotherapy in breast cancer. Ann Surg Oncol. 2021; 28:1320–1325. PMID: 33393046.23. Whitworth P, Beitsch P, Mislowsky A, Pellicane JV, Nash C, Murray M, et al. Chemosensitivity and endocrine sensitivity in clinical luminal breast cancer patients in the prospective Neoadjuvant Breast Registry Symphony Trial (NBRST) predicted by molecular subtyping. Ann Surg Oncol. 2017; 24:669–675.24. Potter DA, Herrera-Ponzanelli CA, Hinojosa D, Castillo R, Hernandez-Cruz I, Arrieta VA, et al. Recent advances in neoadjuvant therapy for breast cancer. Fac Rev. 2021; 10:2. PMID: 33659921.25. Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat. 2007; 105 Suppl 1(Suppl 1):33–43. PMID: 17912634.26. National Comprehensive Cancer Network (NCCN). National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Breast Cancer. NCCN;2022.27. Naffouje SA, Sabesan A, Hoover SJ, Lee MC, Laronga C. Surgical management of the axilla of HER2+ breast cancer in the Z1071 era: a propensity-score-matched analysis of the NCDB. Ann Surg Oncol. 2021; 28:8777–8788. PMID: 34258723.28. Henke G, Knauer M, Ribi K, Hayoz S, Gérard MA, Ruhstaller T, et al. Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials. 2018; 19:667. PMID: 30514362.29. Choy N, Lipson J, Porter C, Ozawa M, Kieryn A, Pal S, et al. Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann Surg Oncol. 2015; 22:377–382. PMID: 25164040.30. Jatoi I, Benson JR, Toi M. De-escalation of axillary surgery in early breast cancer. Lancet Oncol. 2016; 17:e430–e441. PMID: 27733269.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrasonography for Staging Axillary Lymph Node in Breast Cancer Patients
- Ipsilateral Breast Tumor Recurrence with Metachronous Contralateral Axillary Lymph Node Metastasis after Breast-Conserving Surgery with Axillary Lymph Node Dissection
- A Recurrence of Ovarian Carcinoma Presenting as Only Axillary Lymphatic Metastasis: A Case Report
- Predictive Factors Affecting Axillary Lymph Node Metastasis in Patients with Invasive Breast Carcinoma of 1 cm or Less
- Incidence of Axillary Lymph Node Metastases in T1 Breast Cancer