J Korean Med Sci.
2023 Jul;38(28):e216. 10.3346/jkms.2023.38.e216.
Efficacy of Antiviral Prophylaxis up to 6 or 12 Months From Completion of Rituximab in Resolved Hepatitis B Patients: A Multicenter, Randomized Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 2Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 3Division of Hematology-Oncology, Department of Internal Medicine, Konkuk University Medical Center, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- KMID: 2544475
- DOI: http://doi.org/10.3346/jkms.2023.38.e216
Abstract
- Background
Rituximab occasionally induces reactivation of hepatitis B virus (HBV) in patients with resolved HBV, at times with fatal consequences. The optimal duration of prophylactic antiviral therapy in this situation is unclear. We aimed to investigate the difference in HBV reactivation according to the duration of prophylactic tenofovir disoproxil fumarate (TDF) in patients with resolved HBV and receiving rituximab.
Methods
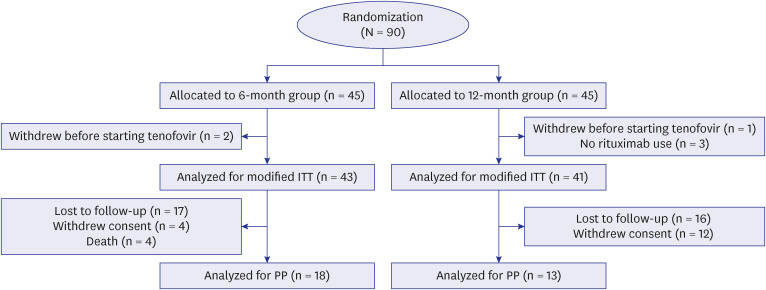
A multicenter, randomized, open-label, prospective study was conducted in hepatitis B surface antigen-negative and anti-HBc-positive non-Hodgkin’s lymphoma patients treated with rituximab-based chemotherapy. A total of 90 patients were randomized and received prophylactic TDF from the initiation of rituximab until 6 months (the 6-month group) or 12 months (the 12-month group) after the completion of rituximab. The primary outcome was the difference in HBV reactivation and the secondary outcomes were the difference in hepatitis flare and adverse events between the two groups.
Results
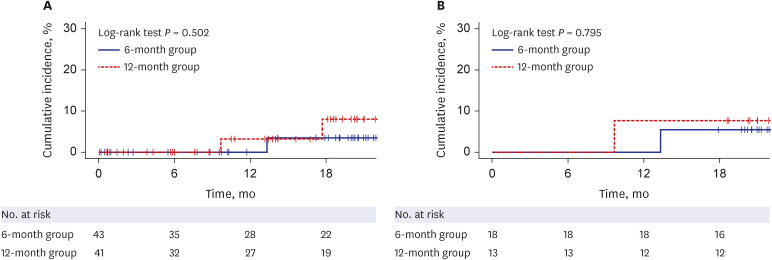
In an intention to treat (ITT) analysis, HBV reactivation occurred in 1 of 43 patients (2.3%; 95% confidence interval [CI], 0.41–12%) at a median of 13.3 months in the 6-month group and 2 of 41 patients (4.9%; 95% CI, 1.4–16%) at a median of 13.7 months in the 12-month group. In a per protocol (PP) analysis, HBV reactivation occurred in 1 of 18 patients (5.6%; 95% CI, 0.99–26%) at 13.3 months in the 6-month group and 1 of 13 patients (7.7%; 95% CI, 1.4–33%) at 9.7 months in the 12-month group. The cumulative incidence of HBV reactivation was not significantly different between the two groups in ITT and PP analyses (P = 0.502 and 0.795, respectively). The occurrence of adverse events was not significantly different between the two groups in ITT (9.3% in the 6-month group, 22.0% in the 12-month group, P = 0.193) and PP analyses (5.6% in the 6-month group, 7.7% in the 12-month group, P > 0.999).
Conclusion
Prophylactic TDF up to 6 months after completion of rituximab-based chemotherapy is sufficient in terms of the efficacy and safety of reducing HBV reactivation in patients with resolved HBV. Trial Registration: ClinicalTrials.gov Identifier: NCT02585947
Keyword
Figure
Reference
-
1. Lau JY, Lai CL, Lin HJ, Lok AS, Liang RH, Wu PC, et al. Fatal reactivation of chronic hepatitis B virus infection following withdrawal of chemotherapy in lymphoma patients. Q J Med. 1989; 73(270):911–917. PMID: 2629023.2. Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991; 100(1):182–188. PMID: 1983820.3. Mason AL, Xu L, Guo L, Kuhns M, Perrillo RP. Molecular basis for persistent hepatitis B virus infection in the liver after clearance of serum hepatitis B surface antigen. Hepatology. 1998; 27(6):1736–1742. PMID: 9620351.4. Murakami Y, Minami M, Daimon Y, Okanoue T. Hepatitis B virus DNA in liver, serum, and peripheral blood mononuclear cells after the clearance of serum hepatitis B virus surface antigen. J Med Virol. 2004; 72(2):203–214. PMID: 14695661.5. Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000; 62(3):299–307. PMID: 11055239.6. Conjeevaram HS, Lok AS. Occult hepatitis B virus infection: a hidden menace? Hepatology. 2001; 34(1):204–206. PMID: 11431752.7. Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009; 49(5):Suppl. S156–S165. PMID: 19399803.8. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017; 152(6):1297–1309. PMID: 28219691.9. Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014; 59(6):2092–2100. PMID: 24002804.10. Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, et al. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014; 32(33):3736–3743. PMID: 25287829.11. Pan J, Yao T, Cheng H, Zhu Y, Wang Y. B lymphocyte-mediated humoral immunity in the pathogenesis of chronic hepatitis B infection. Liver Res. 2020; 4(3):124–128.12. Onrust SV, Lamb HM, Balfour JA. Rituximab. Drugs. 1999; 58(1):79–88. PMID: 10439931.13. Bowzyk Al-Naeeb A, Ajithkumar T, Behan S, Hodson DJ. Non-Hodgkin lymphoma. BMJ. 2018; 362:k3204. PMID: 30135071.14. Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol. 2013; 57(3):209–214. PMID: 23562041.15. Tsutsumi Y, Kanamori H, Mori A, Tanaka J, Asaka M, Imamura M, et al. Reactivation of hepatitis B virus with rituximab. Expert Opin Drug Saf. 2005; 4(3):599–608. PMID: 15934864.16. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007; 45(2):507–539. PMID: 17256718.17. Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998; 339(2):61–68. PMID: 9654535.18. Andreone P, Caraceni P, Grazi GL, Belli L, Milandri GL, Ercolani G, et al. Lamivudine treatment for acute hepatitis B after liver transplantation. J Hepatol. 1998; 29(6):985–989. PMID: 9875646.19. Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004; 22(5):927–934. PMID: 14990649.20. Kohrt HE, Ouyang DL, Keeffe EB. Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2006; 24(7):1003–1016. PMID: 16984494.21. Li YH, He YF, Jiang WQ, Wang FH, Lin XB, Zhang L, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer. 2006; 106(6):1320–1325. PMID: 16470607.22. European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017; 67(2):370–398. PMID: 28427875.23. Terrault NA, Lok AS, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018; 67(4):1560–1599. PMID: 29405329.24. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016; 10(1):1–98.25. Yim HJ, Kim JH, Park JY, Yoon EL, Park H, Kwon JH, et al. Comparison of clinical practice guidelines for the management of chronic hepatitis B: When to start, when to change, and when to stop. Clin Mol Hepatol. 2020; 26(4):411–429. PMID: 32854458.26. Xu K, Liu LM, Farazi PA, Wang H, Rochling FA, Watanabe-Galloway S, et al. Adherence and perceived barriers to oral antiviral therapy for chronic hepatitis B. Glob Health Action. 2018; 11(1):1433987. PMID: 29447614.27. Lee SB, Jeong J, Park JH, Jung SW, Jeong ID, Bang SJ, et al. Low-level viremia and cirrhotic complications in patients with chronic hepatitis B according to adherence to entecavir. Clin Mol Hepatol. 2020; 26(3):364–375. PMID: 32466635.28. Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol. 2017; 9(5):227–241. PMID: 28261380.29. McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998; 16(8):2825–2833. PMID: 9704735.30. Koskinas JS, Deutsch M, Adamidi S, Skondra M, Tampaki M, Alexopoulou A, et al. The role of tenofovir in preventing and treating hepatitis B virus (HBV) reactivation in immunosuppressed patients. A real life experience from a tertiary center. Eur J Intern Med. 2014; 25(8):768–771. PMID: 25037900.31. Casagrande JT, Pike MC, Smith PG. An improved approximate formula for calculating sample sizes for comparing two binomial distributions. Biometrics. 1978; 34(3):483–486. PMID: 719125.32. Chow SC, Wang H, Shao J. Sample Size Calculations in Clinical Research. Boca Raton, FL, USA: CRC Press;2007.33. Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013; 31(22):2765–2772. PMID: 23775967.34. Simon R. Confidence intervals for reporting results of clinical trials. Ann Intern Med. 1986; 105(3):429–435. PMID: 3740683.35. Papp KA, Fonjallaz P, Casset-Semanaz F, Krueger JG, Wittkowski KM. Analytical approaches to reporting long-term clinical trial data. Curr Med Res Opin. 2008; 24(7):2001–2008. PMID: 18534049.36. Shibolet O, Ilan Y, Gillis S, Hubert A, Shouval D, Safadi R. Lamivudine therapy for prevention of immunosuppressive-induced hepatitis B virus reactivation in hepatitis B surface antigen carriers. Blood. 2002; 100(2):391–396. PMID: 12091327.37. Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009; 27(4):605–611. PMID: 19075267.38. Buti M, Manzano ML, Morillas RM, García-Retortillo M, Martín L, Prieto M, et al. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: the Preblin study. PLoS One. 2017; 12(9):e0184550. PMID: 28898281.39. Masarone M, De Renzo A, La Mura V, Sasso FC, Romano M, Signoriello G, et al. Management of the HBV reactivation in isolated HBcAb positive patients affected with non Hodgkin lymphoma. BMC Gastroenterol. 2014; 14(1):31. PMID: 24533834.40. Kusumoto S, Arcaini L, Hong X, Jin J, Kim WS, Kwong YL, et al. Risk of HBV reactivation in patients with B-cell lymphomas receiving obinutuzumab or rituximab immunochemotherapy. Blood. 2019; 133(2):137–146. PMID: 30341058.41. Buti M, Riveiro-Barciela M, Esteban R. Long-term safety and efficacy of nucleo(t)side analogue therapy in hepatitis B. Liver Int. 2018; 38(Suppl 1):84–89.42. Tuttleman JS, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986; 47(3):451–460. PMID: 3768961.43. Yang W, Summers J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor. J Virol. 1999; 73(12):9710–9717. PMID: 10559280.44. Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996; 2(10):1104–1108. PMID: 8837608.45. Inoue T, Tanaka Y. Novel biomarkers for the management of chronic hepatitis B. Clin Mol Hepatol. 2020; 26(3):261–279. PMID: 32536045.46. Wu JW, Kao JH, Tseng TC. Three heads are better than two: hepatitis B core-related antigen as a new predictor of hepatitis B virus-related hepatocellular carcinoma. Clin Mol Hepatol. 2021; 27(4):524–534. PMID: 33618507.47. Jang JW, Kim JS, Kim HS, Tak KY, Nam H, Sung PS, et al. Persistence of intrahepatic hepatitis B virus DNA integration in patients developing hepatocellular carcinoma after hepatitis B surface antigen seroclearance. Clin Mol Hepatol. 2021; 27(1):207–218. PMID: 33317255.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatitis B virus reactivation in a liver transplant recipient using hepatitis B immunoglobulin plus antiviral drug
- Fulminant Hepatic Failure with Hepatitis B Virus Reactivation after Rituximab Treatment in a Patient with Resolved Hepatitis B
- A Case of Acute Hepatitis B by Occult HBV Infection without HbsAg Seroconversion
- A Case of Reactivation of Hepatitis B and Fulminant Hepatitis which developed 3 months following Chemotherapy Including Rituximab in a Patient with Lymphoma
- Prophylaxis Against Hepatitis B Recurrence Following Liver Transplantation