Korean J Physiol Pharmacol.
2023 Jul;27(4):417-426. 10.4196/kjpp.2023.27.4.417.
Monitoring trafficking and expression of hemagglutinin-tagged transient receptor potential melastatin 4 channel in mammalian cells
- Affiliations
-
- 1Brain Science Institute, Korea Institute of Science and Technology (KIST), Seoul 02792, Korea
- 2Department of Physiology, College of Medicine, Gyeongsang National University, Jinju 52727, Korea
- KMID: 2544143
- DOI: http://doi.org/10.4196/kjpp.2023.27.4.417
Abstract
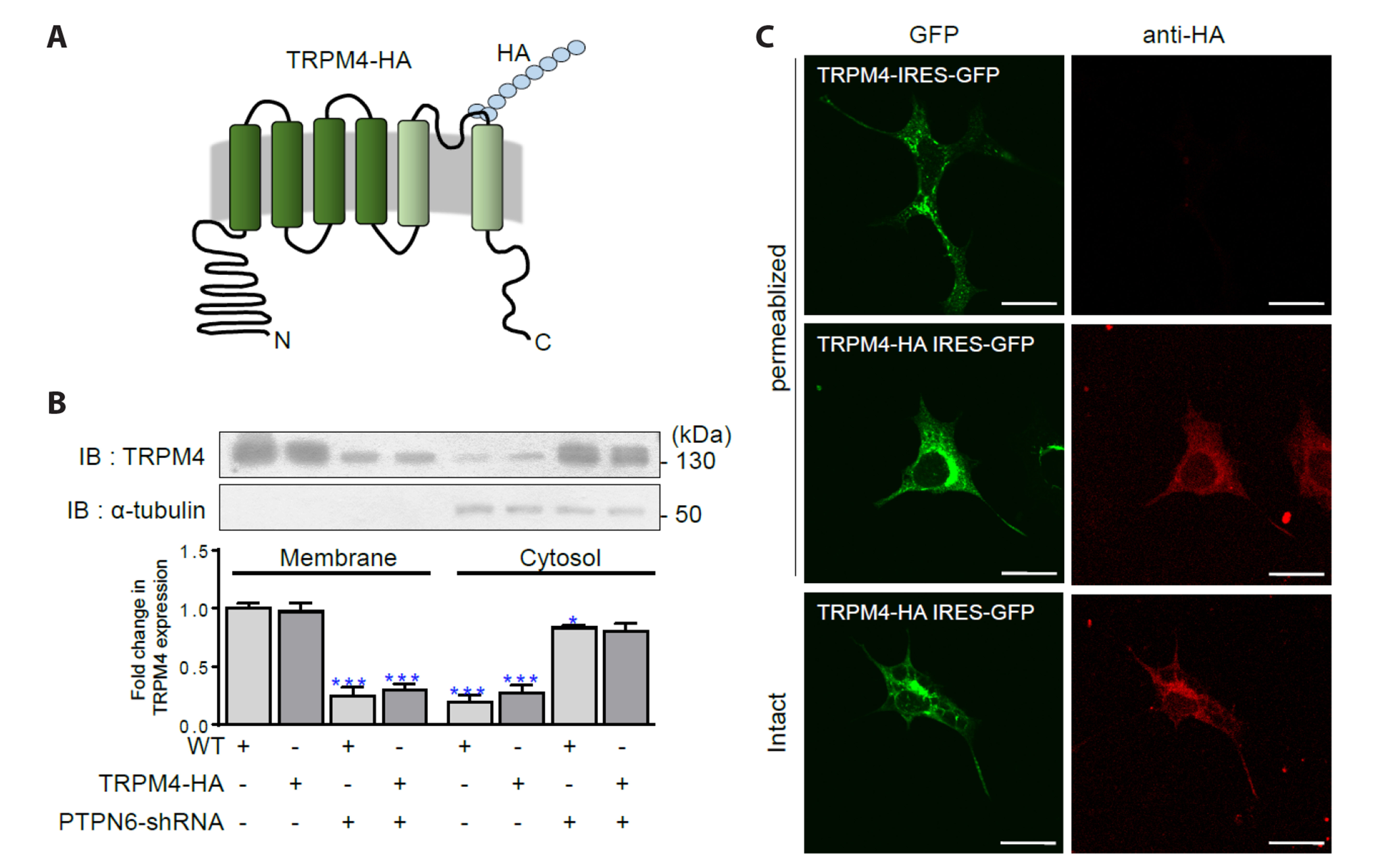
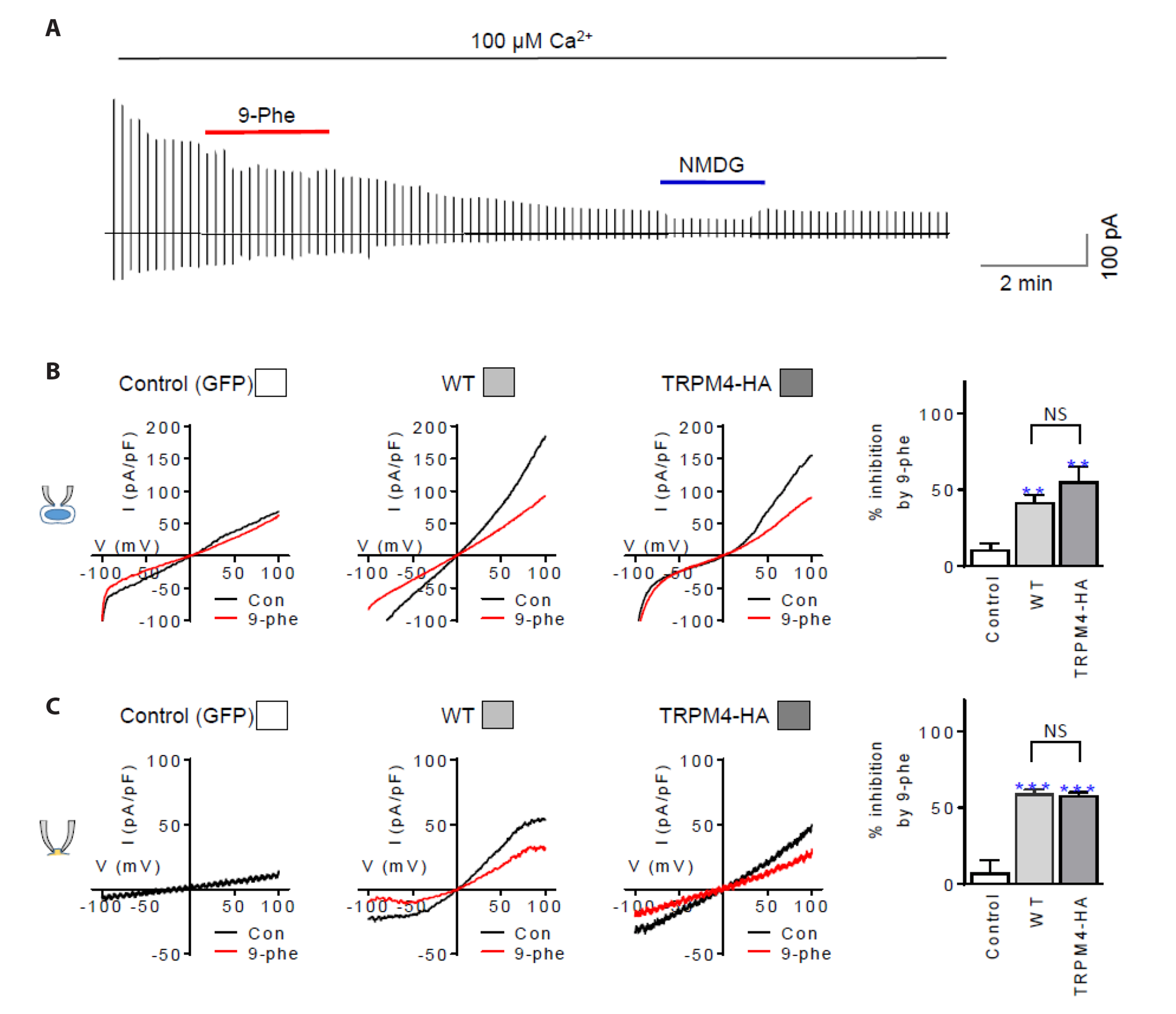
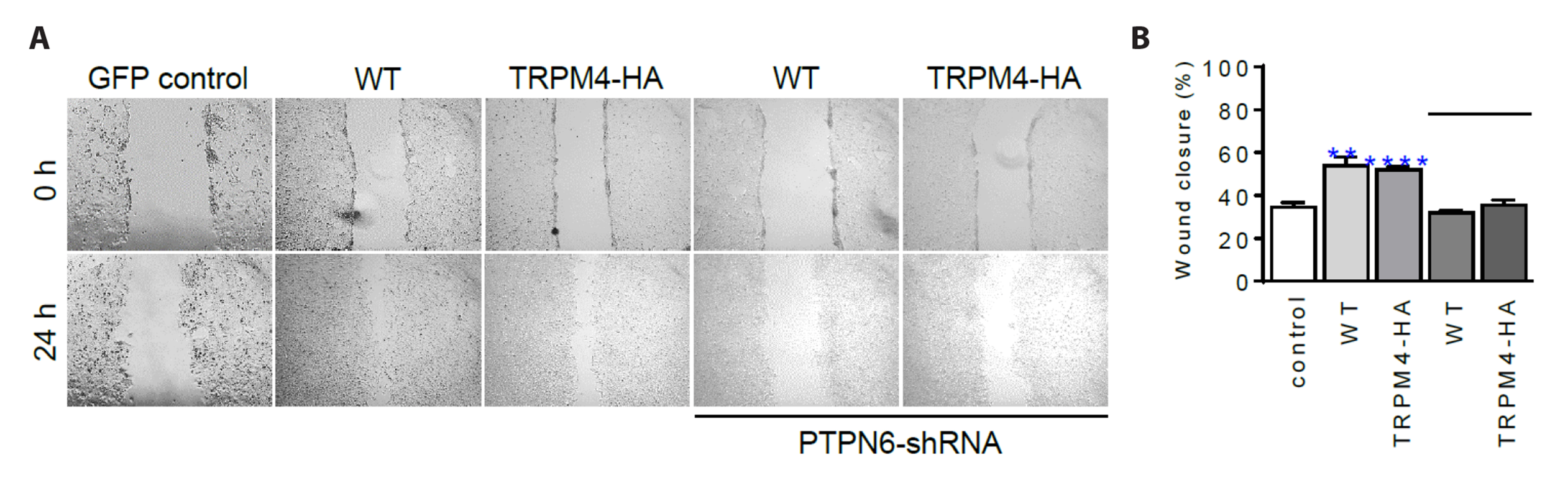
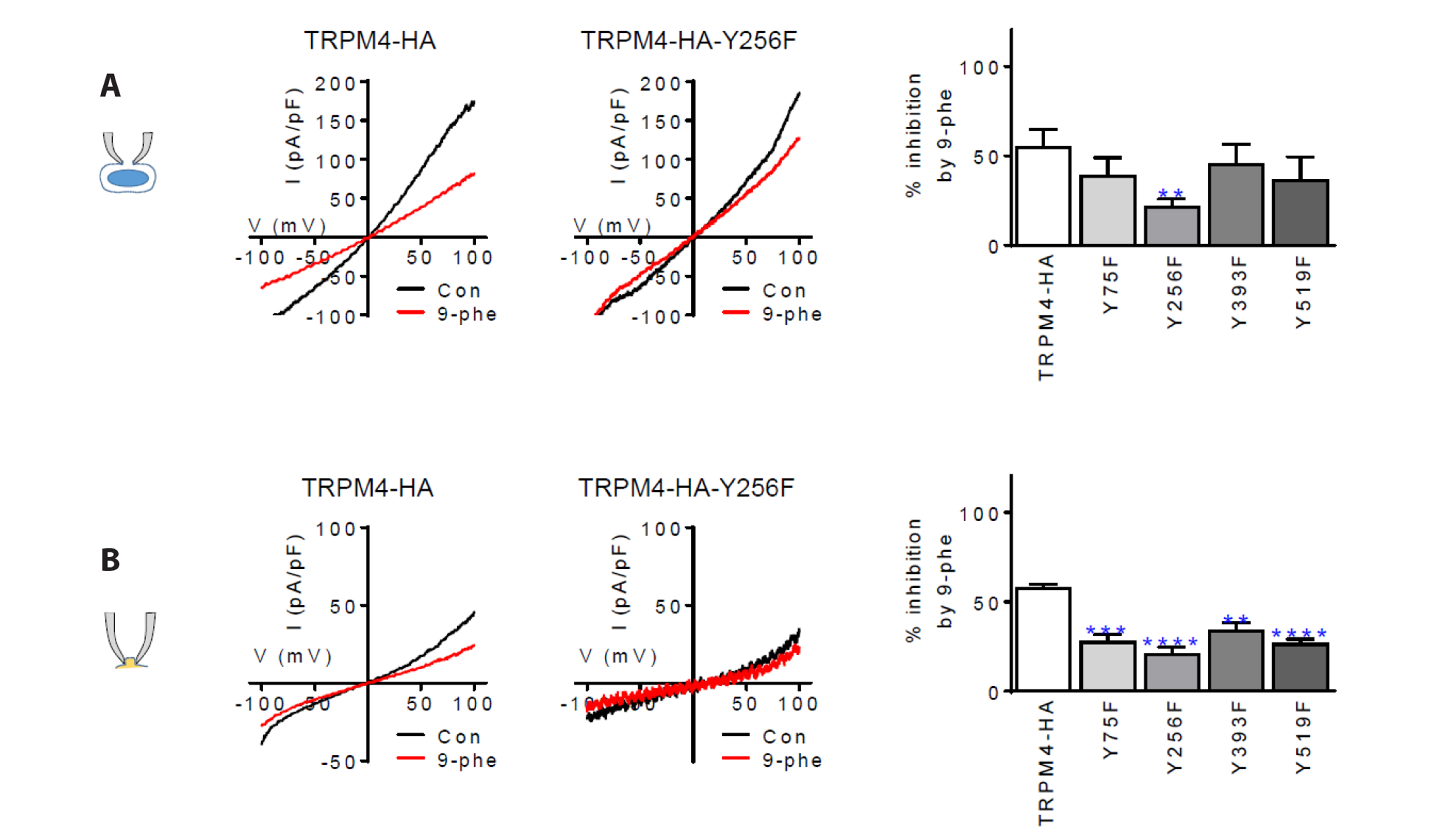
- The TRPM4 gene encodes a Ca2+ -activated monovalent cation channel called transient receptor potential melastatin 4 (TRPM4) that is expressed in various tissues. Dysregulation or abnormal expression of TRPM4 has been linked to a range of diseases. We introduced the hemagglutinin (HA) tag into the extracellular S6 loop of TRPM4, resulting in an HA-tagged version called TRPM4-HA. This TRPM4-HA was developed to investigate the purification, localization, and function of TRPM4 in different physiological and pathological conditions. TRPM4-HA was successfully expressed in the intact cell membrane and exhibited similar electrophysiological properties, such as the current-voltage relationship, rapid desensitization, and current size, compared to the wild-type TRPM4. The presence of the TRPM4 inhibitor 9-phenanthrol did not affect these properties. Furthermore, a wound-healing assay showed that TRPM4-HA induced cell proliferation and migration, similar to the native TRPM4. Co-expression of protein tyrosine phosphatase, non-receptor type 6 (PTPN6 or SHP-1) with TRPM4-HA led to the translocation of TRPM4-HA to the cytosol. To investigate the interaction between PTPN6 and tyrosine residues of TRPM4 in enhancing channel activity, we generated four mutants in which tyrosine (Y) residues were substituted with phenylalanine (F) at the N-terminus of TRPM4. The YF mutants displayed properties and functions similar to TRPM4-HA, except for the Y256F mutant, which showed resistance to 9-phenanthrol, suggesting that Y256 may be involved in the binding site for 9-phenanthrol. Overall, the creation of HA-tagged TRPM4 provides researchers with a valuable tool to study the role of TRPM4 in different conditions and its potential interactions with other proteins, such as PTPN6.
Keyword
Figure
Reference
-
1. Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. 2002; TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 109:397–407. DOI: 10.1016/S0092-8674(02)00719-5. PMID: 12015988.
Article2. Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V. 2003; Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 278:30813–30820. DOI: 10.1074/jbc.M305127200. PMID: 12799367.
Article3. Murakami M, Xu F, Miyoshi I, Sato E, Ono K, Iijima T. 2003; Identification and characterization of the murine TRPM4 channel. Biochem Biophys Res Commun. 307:522–528. DOI: 10.1016/S0006-291X(03)01186-0. PMID: 12893253.
Article4. Jang Y, Lee Y, Kim SM, Yang YD, Jung J, Oh U. 2012; Quantitative analysis of TRP channel genes in mouse organs. Arch Pharm Res. 35:1823–1830. DOI: 10.1007/s12272-012-1016-8. PMID: 23139135.
Article5. Guinamard R, Bouvagnet P, Hof T, Liu H, Simard C, Sallé L. 2015; TRPM4 in cardiac electrical activity. Cardiovasc Res. 108:21–30. DOI: 10.1093/cvr/cvv213. PMID: 26272755.
Article6. Wang H, Xu Z, Lee BH, Vu S, Hu L, Lee M, Bu D, Cao X, Hwang S, Yang Y, Zheng J, Lin Z. 2019; Gain-of-function mutations in TRPM4 activation gate cause progressive symmetric erythrokeratodermia. J Invest Dermatol. 139:1089–1097. DOI: 10.1016/j.jid.2018.10.044. PMID: 30528822.
Article7. Çoban G, Yildiz P, Doğan B, Şahin N, Gücin Z. 2021; Expression of transient receptor potential melastatin 4 in differential diagnosis of eosinophilic renal tumors. Mol Clin Oncol. 15:230. DOI: 10.3892/mco.2021.2393. PMID: 34631055. PMCID: PMC8461610.
Article8. Kappel S, Stokłosa P, Hauert B, Ross-Kaschitza D, Borgström A, Baur R, Galván JA, Zlobec I, Peinelt C. 2019; TRPM4 is highly expressed in human colorectal tumor buds and contributes to proliferation, cell cycle, and invasion of colorectal cancer cells. Mol Oncol. 13:2393–2405. DOI: 10.1002/1878-0261.12566. PMID: 31441200. PMCID: PMC6822246. PMID: 1593c9ef94da47ea9b0e9396bb130ad4.
Article9. Sagredo AI, Sagredo EA, Cappelli C, Báez P, Andaur RE, Blanco C, Tapia JC, Echeverría C, Cerda O, Stutzin A, Simon F, Marcelain K, Armisén R. 2018; TRPM4 regulates Akt/GSK3-β activity and enhances β-catenin signaling and cell proliferation in prostate cancer cells. Mol Oncol. 12:151–165. DOI: 10.1002/1878-0261.12100. PMID: 28614631. PMCID: PMC5792731. PMID: 39b1d5fb4e064e63b1ddea25c367a8f3.
Article10. Cáceres M, Ortiz L, Recabarren T, Romero A, Colombo A, Leiva-Salcedo E, Varela D, Rivas J, Silva I, Morales D, Campusano C, Almarza O, Simon F, Toledo H, Park KS, Trimmer JS, Cerda O. 2015; TRPM4 is a novel component of the adhesome required for focal adhesion disassembly, migration and contractility. PLoS One. 10:e0130540. DOI: 10.1371/journal.pone.0130540. PMID: 26110647. PMCID: PMC4482413. PMID: 14e94f17630944cca89bcbf1c358b2f2.
Article11. Launay P, Cheng H, ivatsan S Sr, Penner R, Fleig A, Kinet JP. 2004; TRPM4 regulates calcium oscillations after T cell activation. Science. 306:1374–1377. DOI: 10.1126/science.1098845. PMID: 15550671.
Article12. Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M. 2007; Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 8:312–320. DOI: 10.1038/ni1441. PMID: 17293867.
Article13. Barbet G, Demion M, Moura IC, Serafini N, Léger T, Vrtovsnik F, Monteiro RC, Guinamard R, Kinet JP, Launay P. 2008; The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol. 9:1148–1156. DOI: 10.1038/ni.1648. PMID: 18758465. PMCID: PMC2956271.
Article14. Shimizu T, Owsianik G, Freichel M, Flockerzi V, Nilius B, Vennekens R. 2009; TRPM4 regulates migration of mast cells in mice. Cell Calcium. 45:226–232. DOI: 10.1016/j.ceca.2008.10.005. PMID: 19046767.15. Gao Y, Liao P. 2019; TRPM4 channel and cancer. Cancer Lett. 454:66–69. DOI: 10.1016/j.canlet.2019.04.012. PMID: 30980865.
Article16. Watanabe H, Murakami M, Ohba T, Ono K, Ito H. 2009; The pathological role of transient receptor potential channels in heart disease. Circ J. 73:419–427. DOI: 10.1253/circj.CJ-08-1153. PMID: 19202304.
Article17. Wang C, Naruse K, Takahashi K. 2018; Role of the TRPM4 channel in cardiovascular physiology and pathophysiology. Cells. 7:62. DOI: 10.3390/cells7060062. PMID: 29914130. PMCID: PMC6025450. PMID: 1bba7c5b29624e5592a2e292f89d525c.
Article18. Otsuka Saito K, Fujita F, Toriyama M, Utami RA, Guo Z, Murakami M, Kato H, Suzuki Y, Okada F, Tominaga M, Ishii KJ. 2023; Roles of TRPM4 in immune responses in keratinocytes and identification of a novel TRPM4-activating agent. Biochem Biophys Res Commun. 654:1–9. DOI: 10.1016/j.bbrc.2023.02.062. PMID: 36871485.
Article19. Holzmann C, Kappel S, Kilch T, Jochum MM, Urban SK, Jung V, Stöckle M, Rother K, Greiner M, Peinelt C. 2015; Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget. 6:41783–41793. DOI: 10.18632/oncotarget.6157. PMID: 26496025. PMCID: PMC4747188.
Article20. Berg KD, Soldini D, Jung M, Dietrich D, Stephan C, Jung K, Dietel M, Vainer B, Kristiansen G. 2016; TRPM4 protein expression in prostate cancer: a novel tissue biomarker associated with risk of biochemical recurrence following radical prostatectomy. Virchows Arch. 468:345–355. DOI: 10.1007/s00428-015-1880-y. PMID: 26590985.
Article21. Sagredo AI, Sagredo EA, Pola V, Echeverría C, Andaur R, Michea L, Stutzin A, Simon F, Marcelain K, Armisén R. 2019; TRPM4 channel is involved in regulating epithelial to mesenchymal transition, migration, and invasion of prostate cancer cell lines. J Cell Physiol. 234:2037–2050. DOI: 10.1002/jcp.27371. PMID: 30343491.
Article22. Ashida S, Nakagawa H, Katagiri T, Furihata M, Iiizumi M, Anazawa Y, Tsunoda T, Takata R, Kasahara K, Miki T, Fujioka T, Shuin T, Nakamura Y. 2004; Molecular features of the transition from prostatic intraepithelial neoplasia (PIN) to prostate cancer: genome-wide gene-expression profiles of prostate cancers and PINs. Cancer Res. 64:5963–5972. DOI: 10.1158/0008-5472.CAN-04-0020. PMID: 15342375.
Article23. Lee DK, Park JY, Yoo JC, Byun EH, Bae YJ, Lee YS, Park N, Kang D, Han J, Park JY, Hwang E, Hong SG. 2018; PTPN6 regulates the cell-surface expression of TRPM4 channels in HEK293 cells. Pflugers Arch. 470:1449–1458. DOI: 10.1007/s00424-018-2161-9. PMID: 29931651.
Article24. Winkler PA, Huang Y, Sun W, Du J, Lü W. 2017; Electron cryo-microscopy structure of a human TRPM4 channel. Nature. 552:200–204. DOI: 10.1038/nature24674. PMID: 29211723.
Article25. Guinamard R, Hof T, Del Negro CA. 2014; The TRPM4 channel inhibitor 9-phenanthrol. Br J Pharmacol. 171:1600–1613. DOI: 10.1111/bph.12582. PMID: 24433510. PMCID: PMC3966741.
Article26. Jarvik JW, Telmer CA. 1998; Epitope tagging. Annu Rev Genet. 32:601–618. DOI: 10.1146/annurev.genet.32.1.601. PMID: 9928493.
Article27. Maue RA. 2007; Understanding ion channel biology using epitope tags: progress, pitfalls, and promise. J Cell Physiol. 213:618–625. DOI: 10.1002/jcp.21259. PMID: 17849449.28. Kennedy ME, Nemec J, Clapham DE. 1996; Localization and interaction of epitope-tagged GIRK1 and CIR inward rectifier K+ channel subunits. Neuropharmacology. 35:831–839. DOI: 10.1016/0028-3908(96)00132-3. PMID: 8938714.
Article29. Peters KW, Qi J, Johnson JP, Watkins SC, Frizzell RA. 2001; Role of snare proteins in CFTR and ENaC trafficking. Pflugers Arch. 443 Suppl 1:S65–69. DOI: 10.1007/s004240100647. PMID: 11845306.
Article30. Czech MP, Chawla A, Woon CW, Buxton J, Armoni M, Tang W, Joly M, Corvera S. 1993; Exofacial epitope-tagged glucose transporter chimeras reveal COOH-terminal sequences governing cellular localization. J Cell Biol. 123:127–135. DOI: 10.1083/jcb.123.1.127. PMID: 8408193. PMCID: PMC2119811.
Article31. Wadzinski BE, Eisfelder BJ, Peruski LF Jr, Mumby MC, Johnson GL. 1992; NH2-terminal modification of the phosphatase 2A catalytic subunit allows functional expression in mammalian cells. J Biol Chem. 267:16883–16888. DOI: 10.1016/S0021-9258(18)41867-4. PMID: 1380955.
Article32. Kast C, Canfield V, Levenson R, Gros P. 1996; Transmembrane organization of mouse P-glycoprotein determined by epitope insertion and immunofluorescence. J Biol Chem. 271:9240–9248. DOI: 10.1074/jbc.271.16.9240. PMID: 8621583.
Article33. Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. 2006; TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem. 281:13588–13595. DOI: 10.1074/jbc.M512205200. PMID: 16537542.34. Armisén R, Marcelain K, Simon F, Tapia JC, Toro J, Quest AF, Stutzin A. 2011; TRPM4 enhances cell proliferation through up-regulation of the β-catenin signaling pathway. J Cell Physiol. 226:103–109. DOI: 10.1002/jcp.22310. PMID: 20625999.
Article35. Autzen HE, Myasnikov AG, Campbell MG, Asarnow D, Julius D, Cheng Y. 2018; Structure of the human TRPM4 ion channel in a lipid nanodisc. Science. 359:228–232. DOI: 10.1126/science.aar4510. PMID: 29217581. PMCID: PMC5898196.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Pathophysiologic Roles of TRPM7 Channel
- Changes of Tissue Inhibitors of Metalloproteinase Expression by Transient Receptor Potential Melastatin-Subfamily Member 7 Suppression in Renal Cancer Cells
- The Role of Transient Receptor Potential Channel in Pain
- Inhibition of the Desensitization of Canonical Transient Receptor Potential Channel 5 by Dimethyl Sulfoxide
- pH-mediated Regulation of Pacemaker Activity in Cultured Interstitial Cells of Cajal