Korean J Physiol Pharmacol.
2014 Feb;18(1):15-23. 10.4196/kjpp.2014.18.1.15.
The Pathophysiologic Roles of TRPM7 Channel
- Affiliations
-
- 1Division of Longevity and Biofunctional Medicine, Pusan National University School of Korean Medicine, Yangsan 626-870, Korea. vision@pusan.ac.kr
- 2Department of Physiology, Seoul National University College of Medicine, Seoul 110-799, Korea. insuk@snu.ac.kr
- KMID: 2285486
- DOI: http://doi.org/10.4196/kjpp.2014.18.1.15
Abstract
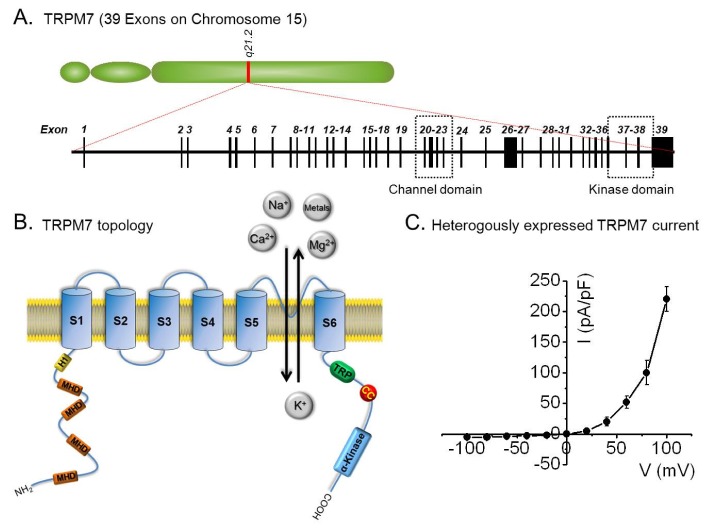
- Transient receptor potential melastatin 7 (TRPM7) is a member of the melastatin-related subfamily and contains a channel and a kinase domain. TRPM7 is known to be associated with cell proliferation, survival, and development. It is ubiquitously expressed, highly permeable to Mg2+ and Ca2+, and its channel activity is negatively regulated by free Mg2+ and Mg-complexed nucleotides. Recent studies have investigated the relationships between TRPM7 and a number of diseases. TRPM7 regulates cell proliferation in several cancers, and is associated with ischemic cell death and vascular smooth muscle cell (VSMC) function. This review discusses the physiologic and pathophysiologic functions and significance of TRPM7 in several diseases.
MeSH Terms
Figure
Reference
-
1. Doyle JL, Stubbs L. Ataxia, arrhythmia and ion-channel gene defects. Trends Genet. 1998; 14:92–98. PMID: 9540405.
Article3. Farias LM, Ocaña DB, Díaz L, Larrea F, Avila-Chávez E, Cadena A, Hinojosa LM, Lara G, Villanueva LA, Vargas C, Hernández-Gallegos E, Camacho-Arroyo I, Dueñas-González A, Pérez-Cárdenas E, Pardo LA, Morales A, Taja-Chayeb L, Escamilla J, Sánchez-Peña C, Camacho J. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004; 64:6996–7001. PMID: 15466192.4. Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda). 2004; 19:285–292. PMID: 15381757.
Article5. Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W. Oncogenic potential of EAG K+ channels. EMBO J. 1999; 18:5540–5547. PMID: 10523298.6. Schwarz EC, Wissenbach U, Niemeyer BA, Strauss B, Philipp SE, Flockerzi V, Hoth M. TRPV6 potentiates calcium-dependent cell proliferation. Cell Calcium. 2006; 39:163–173. PMID: 16356545.
Article7. Sanchez MG, Sanchez AM, Collado B, Malagarie-Cazenave S, Olea N, Carmena MJ, Prieto JC, Diaz-Laviada I I. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol. 2005; 515:20–27. PMID: 15913603.10. Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005; 38:233–252. PMID: 16098585.
Article11. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006; 68:619–647. PMID: 16460286.
Article12. Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011; 12:218. PMID: 21401968.
Article13. Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem. 2011; 28:813–822. PMID: 22178934.
Article14. Yee NS, Chan AS, Yee JD, Yee RK. TRPM7 and TRPM8 ion channels in pancreatic adenocarcinoma: potential roles as cancer biomarkers and targets. Scientifica (Cairo). 2012; 2012:415158. PMID: 24278689.
Article15. Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7-Gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007; 1772:813–821. PMID: 17481860.
Article16. Paravicini TM, Chubanov V, Gudermann T. TRPM7: a unique channel involved in magnesium homeostasis. Int J Biochem Cell Biol. 2012; 44:1381–1384. PMID: 22634382.
Article17. Mederos y Schnitzler M, Wäring J, Gudermann T, Chubanov V. Evolutionary determinants of divergent calcium selectivity of TRPM channels. FASEB J. 2008; 22:1540–1551. PMID: 18073331.18. Mei ZZ, Xia R, Beech DJ, Jiang LH. Intracellular coiled-coil domain engaged in subunit interaction and assembly of melastatin-related transient receptor potential channel 2. J Biol Chem. 2006; 281:38748–38756. PMID: 17060318.
Article19. Bae CY, Sun HS. TRPM7 in cerebral ischemia and potential target for drug development in stroke. Acta Pharmacol Sin. 2011; 32:725–733. PMID: 21552293.
Article20. Yamaguchi H, Matsushita M, Nairn AC, Kuriyan J. Crystal structure of the atypical protein kinase domain of a TRP channel with phosphotransferase activity. Mol Cell. 2001; 7:1047–1057. PMID: 11389851.
Article21. Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003; 121:49–60. PMID: 12508053.
Article22. Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001; 411:590–595. PMID: 11385574.
Article23. Demeuse P, Penner R, Fleig A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen Physiol. 2006; 127:421–434. PMID: 16533898.
Article24. Runnels LW, Yue L, Clapham DE. The TRPM7 channel is inactivated by PIP(2) hydrolysis. Nat Cell Biol. 2002; 4:329–336. PMID: 11941371.
Article25. Yogi A, Callera GE, Antunes TT, Tostes RC, Touyz RM. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J. 2011; 75:237–245. PMID: 21150127.
Article26. Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005; 280:37763–37771. PMID: 16150690.
Article27. Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006; 127:525–537. PMID: 16636202.
Article28. Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002; 119:487–507. PMID: 11981025.29. Parnas M, Peters M, Dadon D, Lev S, Vertkin I, Slutsky I, Minke B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium. 2009; 45:300–309. PMID: 19135721.
Article30. Chen HC, Xie J, Zhang Z, Su LT, Yue L, Runnels LW. Blockade of TRPM7 channel activity and cell death by inhibitors of 5-lipoxygenase. PLoS One. 2010; 5:e11161. PMID: 20567598.
Article31. Chen X, Numata T, Li M, Mori Y, Orser BA, Jackson MF, Xiong ZG, MacDonald JF. The modulation of TRPM7 currents by nafamostat mesilate depends directly upon extracellular concentrations of divalent cations. Mol Brain. 2010; 3:38. PMID: 21122141.
Article32. Chubanov V, Mederos y Schnitzler M, Meißner M, Schäfer S, Abstiens K, Hofmann T, Gudermann T. Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br J Pharmacol. 2012; 166:1357–1376. PMID: 22242975.
Article33. Zierler S, Yao G, Zhang Z, Kuo WC, Pörzgen P, Penner R, Horgen FD, Fleig A. Waixenicin A inhibits cell proliferation through magnesium-dependent block of transient receptor potential melastatin 7 (TRPM7) channels. J Biol Chem. 2011; 286:39328–39335. PMID: 21926172.
Article34. Qin X, Yue Z, Sun B, Yang W, Xie J, Ni E, Feng Y, Mahmood R, Zhang Y, Yue L. Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br J Pharmacol. 2013; 168:1294–1312. PMID: 23145923.
Article35. Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L. Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem. 2007; 282:25817–25830. PMID: 17599911.36. Jiang J, Li M, Yue L. Potentiation of TRPM7 inward currents by protons. J Gen Physiol. 2005; 126:137–150. PMID: 16009728.
Article37. Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res. 2007; 67:10929–10938. PMID: 18006838.
Article38. Numata T, Okada Y. Proton conductivity through the human TRPM7 channel and its molecular determinants. J Biol Chem. 2008; 283:15097–15103. PMID: 18390554.
Article39. Penner R, Fleig A. The Mg2+ and Mg2+-nucleotide-regulated channel-kinase TRPM7. Handb Exp Pharmacol. 2007; (179):313–328. PMID: 17217066.40. Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proc Natl Acad Sci U S A. 2005; 102:11510–11515. PMID: 16051700.
Article41. Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S. Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res. 2006; 26:159–178. PMID: 16777713.
Article42. Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001; 291:1043–1047. PMID: 11161216.
Article43. He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005; 96:207–215. PMID: 15591230.44. Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004; 95:403–419. PMID: 15286426.45. Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM. Defective skeletogenesis with kidney stone formation in dwarf zebrafish mutant for trpm7. Curr Biol. 2005; 15:667–671. PMID: 15823540.
Article46. Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003; 115:863–877. PMID: 14697204.
Article47. Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007; 40:849–865. PMID: 18021175.
Article48. Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008; 322:756–760. PMID: 18974357.49. Liu W, Su LT, Khadka DK, Mezzacappa C, Komiya Y, Sato A, Habas R, Runnels LW. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev Biol. 2011; 350:348–357. PMID: 21145885.
Article50. Ryazanova LV, Rondon LJ, Zierler S, Hu Z, Galli J, Yamaguchi TP, Mazur A, Fleig A, Ryazanov AG. TRPM7 is essential for Mg2+ homeostasis in mammals. Nat Commun. 2010; 1:109. PMID: 21045827.51. Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, Yue L, Runnels LW. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006; 281:11260–11270. PMID: 16436382.
Article52. Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K, van Leeuwen FN. TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J. 2006; 25:290–301. PMID: 16407977.
Article53. Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009; 457:901–905. PMID: 19118385.
Article54. Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006; 52:485–496. PMID: 17088214.
Article55. Oancea E, Wolfe JT, Clapham DE. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ Res. 2006; 98:245–253. PMID: 16357306.
Article56. Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006; 99:119–131. PMID: 16857972.
Article57. Sah R, Mesirca P, Mason X, Gibson W, Bates-Withers C, Van den Boogert M, Chaudhuri D, Pu WT, Mangoni ME, Clapham DE. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation. 2013; 128:101–114. PMID: 23734001.58. Sah R, Mesirca P, Van den Boogert M, Rosen J, Mably J, Mangoni ME, Clapham DE. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc Natl Acad Sci U S A. 2013; 110:E3037–E3046. PMID: 23878236.
Article59. Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005; 129:1504–1517. PMID: 16285951.
Article60. Yue D, Wang Y, Xiao JY, Wang P, Ren CS. Expression of TRPC6 in benign and malignant human prostate tissues. Asian J Androl. 2009; 11:541–547. PMID: 19701218.
Article61. Santoni G, Farfariello V, Amantini C. TRPV channels in tumor growth and progression. Adv Exp Med Biol. 2011; 704:947–967. PMID: 21290335.
Article62. Duncan LM, Deeds J, Cronin FE, Donovan M, Sober AJ, Kauffman M, McCarthy JJ. Melastatin expression and prognosis in cutaneous malignant melanoma. J Clin Oncol. 2001; 19:568–576. PMID: 11208852.
Article63. Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res. 2001; 61:3760–3769. PMID: 11325849.64. Dou Y, Li Y, Chen J, Wu S, Xiao X, Xie S, Tang L, Yan M, Wang Y, Lin J, Zhu W, Yan G. Inhibition of cancer cell proliferation by midazolam by targeting transient receptor potential melastatin 7. Oncol Lett. 2013; 5:1010–1016. PMID: 23426784.
Article65. Kim BJ, Park EJ, Lee JH, Jeon JH, Kim SJ, So I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008; 99:2502–2509. PMID: 19032368.
Article66. Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009; 297:C493–C502. PMID: 19515901.
Article67. Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006; 296:2939–2946. PMID: 17190894.
Article68. Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, Cade JE, Gale CP, Burley VJ. Dietary fiber intake and risk of first stroke: a systematic review and meta-analysis. Stroke. 2013; 44:1360–1368. PMID: 23539529.69. Kristián T, Siesjö BK. Calcium in ischemic cell death. Stroke. 1998; 29:705–718. PMID: 9506616.
Article70. Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med (Berl). 2000; 78:3–13. PMID: 10759025.
Article71. MacDonald JF, Xiong ZG, Jackson MF. Paradox of Ca2+ signaling, cell death and stroke. Trends Neurosci. 2006; 29:75–81. PMID: 16376999.72. Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988; 1:623–634. PMID: 2908446.
Article73. Horn J, Limburg M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst Rev. 2000; (2):CD001928. PMID: 10796454.
Article74. Tymianski M, Charlton MP, Carlen PL, Tator CH. Secondary Ca2+ overload indicates early neuronal injury which precedes staining with viability indicators. Brain Res. 1993; 607:319–323. PMID: 7683241.75. Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989; 36:106–112. PMID: 2568579.76. Randall RD, Thayer SA. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992; 12:1882–1895. PMID: 1349638.
Article77. Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies-the need for new approaches. Cerebrovasc Dis. 2004; 17(Suppl 1):153–166. PMID: 14694293.78. Lipski J, Park TI, Li D, Lee SC, Trevarton AJ, Chung KK, Freestone PS, Bai JZ. Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res. 2006; 1077:187–199. PMID: 16483552.
Article79. Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, Golde TE, Orser BA, Macdonald JF, Tymianski M. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009; 12:1300–1307. PMID: 19734892.
Article80. Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, Kastarinen M, Poulter N, Primatesta P, Rodríguez-Artalejo F, Stegmayr B, Thamm M, Tuomilehto J, Vanuzzo D, Vescio F. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. 2003; 289:2363–2369. PMID: 12746359.81. Resnick LM, Laragh JH, Sealey JE, Alderman MH. Divalent cations in essential hypertension. Relations between serum ionized calcium, magnesium, and plasma renin activity. N Engl J Med. 1983; 309:888–891. PMID: 6350880.82. Resnick LM, Gupta RK, Bhargava KK, Gruenspan H, Alderman MH, Laragh JH. Cellular ions in hypertension, diabetes, and obesity. A nuclear magnetic resonance spectroscopic study. Hypertension. 1991; 17:951–957. PMID: 2045175.
Article83. Resnick LM. Cellular calcium and magnesium metabolism in the pathophysiology and treatment of hypertension and related metabolic disorders. Am J Med. 1992; 93:11S–20S. PMID: 1387762.
Article84. Aviv A. Salt consumption, reactive oxygen species and cardiovascular ageing: a hypothetical link. J Hypertens. 2002; 20:555–559. PMID: 11910279.
Article85. Schiffrin EL, Touyz RM. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart Circ Physiol. 2004; 287:H435–H446. PMID: 15277186.
Article86. Rubin H. The logic of the Membrane, Magnesium, Mitosis (MMM) model for the regulation of animal cell proliferation. Arch Biochem Biophys. 2007; 458:16–23. PMID: 16750508.
Article87. Yoshimura M, Oshima T, Matsuura H, Ishida T, Kambe M, Kajiyama G. Extracellular Mg2+ inhibits capacitative Ca2+ entry in vascular smooth muscle cells. Circulation. 1997; 95:2567–2572. PMID: 9184588.88. Touyz RM, He Y, Montezano AC, Yao G, Chubanov V, Gudermann T, Callera GE. Differential regulation of transient receptor potential melastatin 6 and 7 cation channels by ANG II in vascular smooth muscle cells from spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2006; 290:R73–R78. PMID: 16109804.
Article89. Voets T, Nilius B. Modulation of TRPs by PIPs. J Physiol. 2007; 582:939–944. PMID: 17395625.
Article90. Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004; 279:19–25. PMID: 14576148.91. Touyz RM. Transient receptor potential melastatin 6 and 7 channels, magnesium transport, and vascular biology: implications in hypertension. Am J Physiol Heart Circ Physiol. 2008; 294:H1103–H1118. PMID: 18192217.
Article92. Sontia B, Montezano AC, Paravicini T, Tabet F, Touyz RM. Downregulation of renal TRPM7 and increased inflammation and fibrosis in aldosterone-infused mice: effects of magnesium. Hypertension. 2008; 51:915–921. PMID: 18268139.93. Touyz RM, Schiffrin EL. Activation of the Na+-H+ exchanger modulates angiotensin II-stimulated Na+-dependent Mg2+ transport in vascular smooth muscle cells in genetic hypertension. Hypertension. 1999; 34:442–449. PMID: 10489391.94. Touyz RM, Yao G. Inhibitors of Na+/Mg2+ exchange activity attenuate the development of hypertension in angiotensin II-induced hypertensive rats. J Hypertens. 2003; 21:337–344. PMID: 12569264.95. Dietrich A, Mederos Y, Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6-/-mice. Mol Cell Biol. 2005; 25:6980–6989. PMID: 16055711.96. Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londoño JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest. 2010; 120:3267–3279. PMID: 20679729.
Article97. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006; 7:335–346. PMID: 16760914.
Article98. Vennekens R. Emerging concepts for the role of TRP channels in the cardiovascular system. J Physiol. 2011; 589:1527–1534. PMID: 21173080.
Article99. Shaw PJ. Molecular and cellular pathways of neurodegeneration in motor neurone disease. J Neurol Neurosurg Psychiatry. 2005; 76:1046–1057. PMID: 16024877.
Article100. Hara K, Kokubo Y, Ishiura H, Fukuda Y, Miyashita A, Kuwano R, Sasaki R, Goto J, Nishizawa M, Kuzuhara S, Tsuji S. TRPM7 is not associated with amyotrophic lateral sclerosis-parkinsonism dementia complex in the Kii peninsula of Japan. Am J Med Genet B Neuropsychiatr Genet. 2010; 153B:310–313. PMID: 19405049.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TRPM7 Is Essential for RANKL-Induced Osteoclastogenesis
- Higher Expression of TRPM7 Channels in Murine Mature B Lymphocytes than Immature Cells
- Down-regulation of transient receptor potential melastatin member 7 prevents migration and invasion of renal cell carcinoma cells via inactivation of the Src and Akt pathway
- pH-mediated Regulation of Pacemaker Activity in Cultured Interstitial Cells of Cajal
- Changes of Tissue Inhibitors of Metalloproteinase Expression by Transient Receptor Potential Melastatin-Subfamily Member 7 Suppression in Renal Cancer Cells