Korean J Physiol Pharmacol.
2023 Jul;27(4):357-364. 10.4196/kjpp.2023.27.4.357.
Shikonin ameliorates salivary gland damage and inflammation in a mouse model of Sjögren’s syndrome by modulating MAPK signaling pathway
- Affiliations
-
- 1Department of Rheumatology and Immunology, The First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui 233004, China
- KMID: 2544137
- DOI: http://doi.org/10.4196/kjpp.2023.27.4.357
Abstract
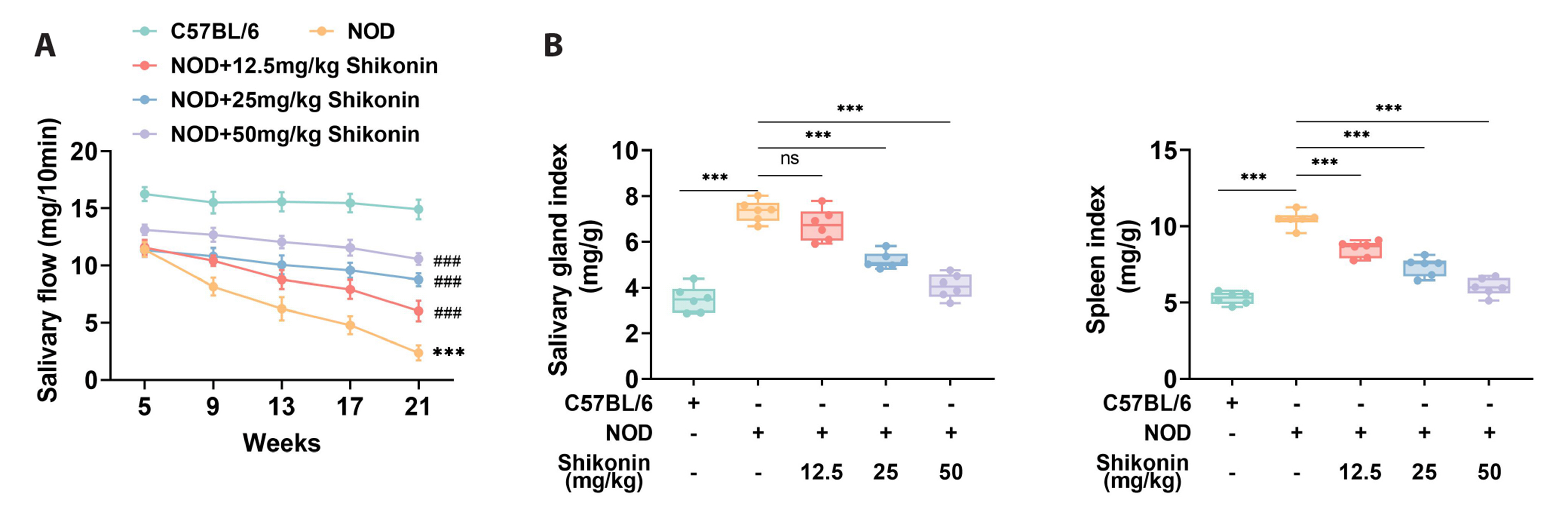
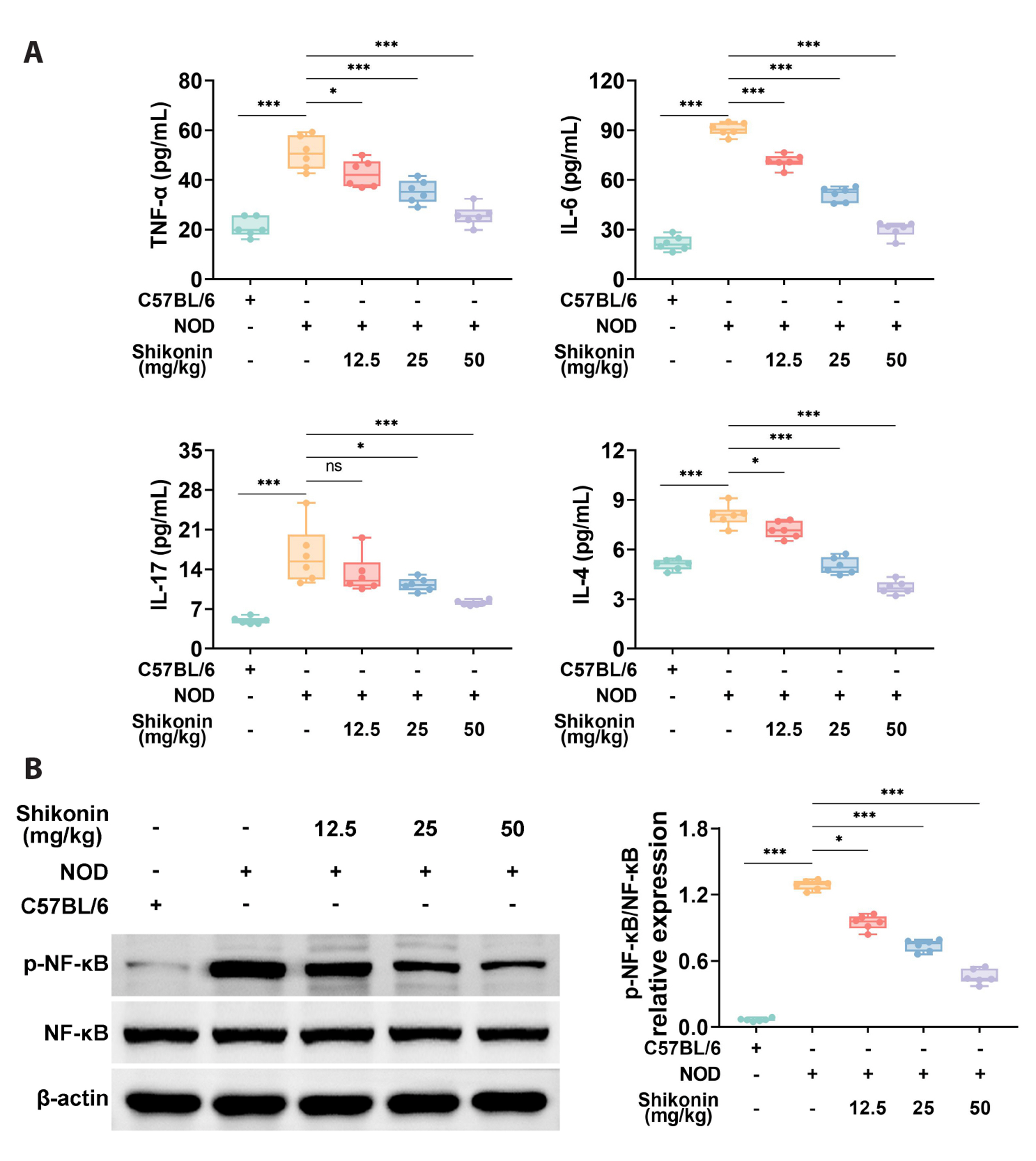
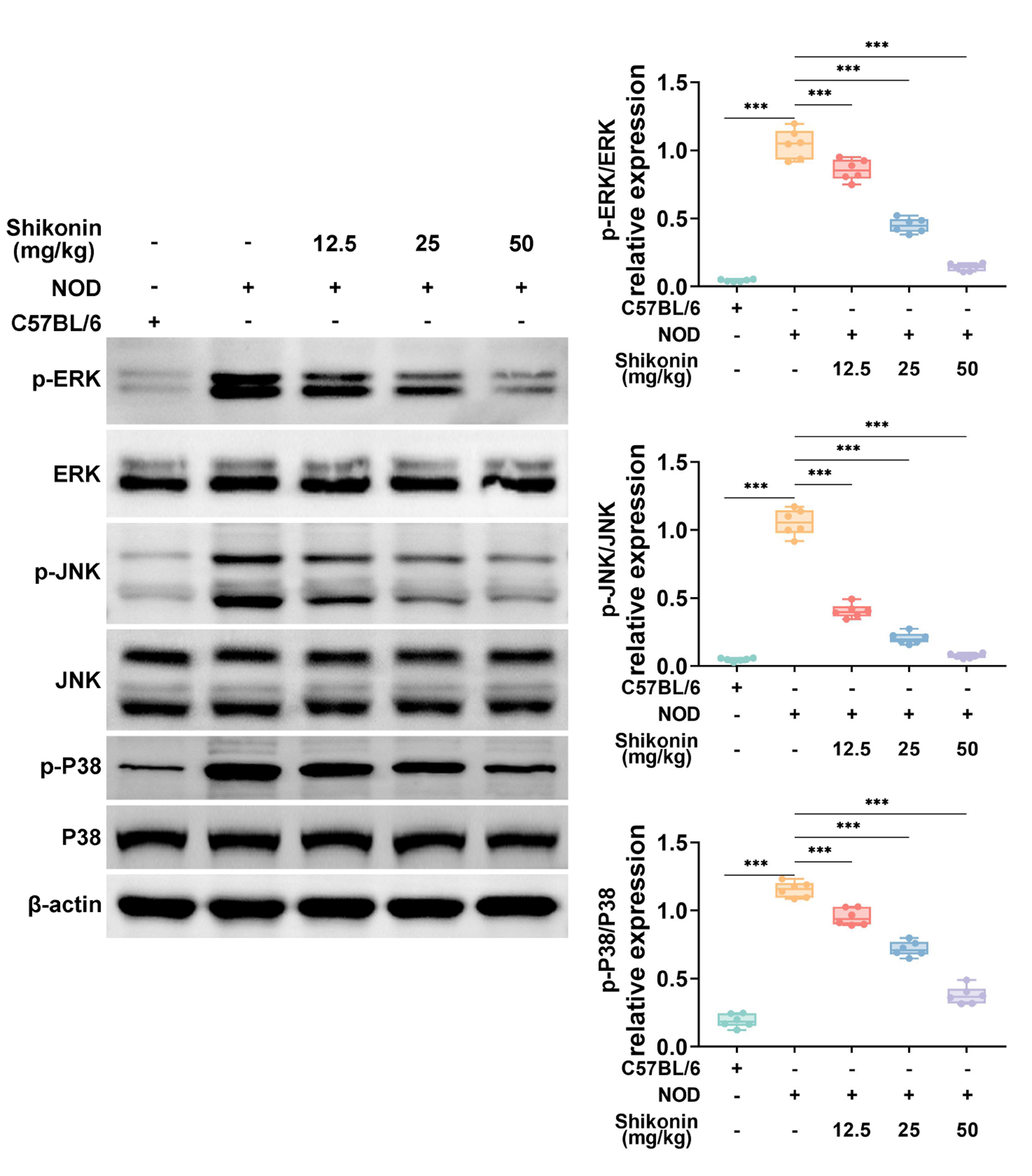
- Sjögren syndrome (SS) is a systemic inflammatory autoimmune disease that involves exocrine glands. Shikonin is extracted from comfrey, which is conventionally used as an anti-tumor, antibacterial, and antiviral drug in China. However, the application of Shikonin in SS remains unreported. This study aimed to verify the potential functions of Shikonin in SS progression. Firstly, non-obese diabetic mice were used as the SS mouse model, with C57BL/6 mice serving as the healthy control. It was demonstrated that the salivary gland damage and inflammation were aggravated in the SS mouse model. Shikonin improved salivary gland function decline and injury in the SS mouse model. Moreover, Shikonin reduced inflammatory cytokines and immune infiltration in the SS mouse model. Further experiments discovered that Shikonin attenuated the MAPK signaling pathway in the SS mouse model. Lastly, inhibition of the MAPK signaling pathway combined with Shikonin treatment further alleviated the symptoms of SS. In conclusion, Shikonin ameliorated salivary gland damage and inflammation in a mouse model of SS by modulating the MAPK signaling pathway. Our findings indicate that Shikonin may be a useful drug for SS treatment.
Keyword
Figure
Reference
-
1. Nakano Y, Yamamoto M, Komatsu K, Yagita M, Fujita M. 2018; Hypertrophic pachymeningitis in Sjögren's syndrome. Intern Med. 57:413–415. DOI: 10.2169/internalmedicine.9406-17. PMID: 29093421. PMCID: PMC5827326.
Article2. Sumida T, Azuma N, Moriyama M, Takahashi H, Asashima H, Honda F, Abe S, Ono Y, Hirota T, Hirata S, Tanaka Y, Shimizu T, Nakamura H, Kawakami A, Sano H, Ogawa Y, Tsubota K, Ryo K, Saito I, Tanaka A, et al. 2018; Clinical practice guideline for Sjögren's syndrome 2017. Mod Rheumatol. 28:383–408. DOI: 10.1080/14397595.2018.1438093. PMID: 29409370.
Article3. Imgenberg-Kreuz J, Sandling JK, Nordmark G. 2018; Epigenetic alterations in primary Sjögren's syndrome - an overview. Clin Immunol. 196:12–20. DOI: 10.1016/j.clim.2018.04.004. PMID: 29649576.
Article4. Verstappen GM, Meiners PM, Corneth OBJ, Visser A, Arends S, Abdulahad WH, Hendriks RW, Vissink A, Kroese FGM, Bootsma H. 2017; Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjögren's syndrome. Arthritis Rheumatol. 69:1850–1861. DOI: 10.1002/art.40165. PMID: 28564491.
Article5. Nakamura H, Hasegawa H, Sasaki D, Takatani A, Shimizu T, Kurushima S, Horai Y, Nakashima Y, Nakamura T, Fukuoka J, Kawakami A. 2018; Detection of human T lymphotropic virus type-I bZIP factor and tax in the salivary glands of Sjögren's syndrome patients. Clin Exp Rheumatol. 36 Suppl 112:51–60.6. Ranta N, Valli A, Grönholm A, Silvennoinen O, Isomäki P, Pesu M, Pertovaara M. 2018; Proprotein convertase enzyme FURIN is upregulated in primary Sjögren's syndrome. Clin Exp Rheumatol. 36 Suppl 112:47–50.7. Song C, Adili A, Kari A, Abuduhaer A. 2021; FSTL1 aggravates sepsis-induced acute kidney injury through regulating TLR4/MyD88/NF-κB pathway in newborn rats. Signa Vitae. 17:167–173. DOI: 10.22514/sv.2021.071.8. Matsui K, Sano H. 2017; T helper 17 cells in primary Sjögren's syndrome. J Clin Med. 6:65. DOI: 10.3390/jcm6070065. PMID: 28678161. PMCID: PMC5532573. PMID: 9fcdfb9c61ce465aa221b7dbe1bc37ba.
Article9. Nicaise C, Weichselbaum L, Schandene L, Gangji V, Dehavay F, Bouchat J, Balau B, Vogl T, Soyfoo MS. 2017; Phagocyte-specific S100A8/A9 is upregulated in primary Sjögren's syndrome and triggers the secretion of pro-inflammatory cytokines in vitro. Clin Exp Rheumatol. 35:129–136. PMID: 27749214.10. Sun Q, Gong T, Liu M, Ren S, Yang H, Zeng S, Zhao H, Chen L, Ming T, Meng X, Xu H. 2022; Shikonin, a naphthalene ingredient: therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine. 94:153805. DOI: 10.1016/j.phymed.2021.153805. PMID: 34749177.11. Yadav S, Sharma A, Nayik GA, Cooper R, Bhardwaj G, Sohal HS, Mutreja V, Kaur R, Areche FO, AlOudat M, Shaikh AM, Kovács B, Mohamed Ahmed AE. 2022; Review of shikonin and derivatives: isolation, chemistry, biosynthesis, pharmacology and toxicology. Front Pharmacol. 13:905755. DOI: 10.3389/fphar.2022.905755. PMID: 35847041. PMCID: PMC9283906. PMID: 60cd9eec9c384004aaf944a7253df391.
Article12. Cao HH, Liu DY, Lai YC, Chen YY, Yu LZ, Shao M, Liu JS. 2020; Inhibition of the STAT3 signaling pathway contributes to the anti-melanoma activities of shikonin. Front Pharmacol. 11:748. DOI: 10.3389/fphar.2020.00748. PMID: 32536866. PMCID: PMC7267064. PMID: 11b0e8a5b01243ac8a37752c12988778.13. Huang XY, Fu HL, Tang HQ, Yin ZQ, Zhang W, Shu G, Yin LZ, Zhao L, Yan XR, Lin JC. 2020; Optimization extraction of shikonin using ultrasound-assisted response surface methodology and antibacterial studies. Evid Based Complement Alternat Med. 2020:1208617. DOI: 10.1155/2020/1208617. PMID: 32802111. PMCID: PMC7411493.
Article14. Figat R, Zgadzaj A, Geschke S, Sieczka P, Pietrosiuk A, Sommer S, Skrzypczak A. 2021; Cytotoxicity and antigenotoxicity evaluation of acetylshikonin and shikonin. Drug Chem Toxicol. 44:140–147. DOI: 10.1080/01480545.2018.1536710. PMID: 30574814.
Article15. Guo H, Sun J, Li D, Hu Y, Yu X, Hua H, Jing X, Chen F, Jia Z, Xu J. 2019; Shikonin attenuates acetaminophen-induced acute liver injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother. 112:108704. DOI: 10.1016/j.biopha.2019.108704. PMID: 30818140.
Article16. Liu S, Yan W, Hu Y, Wu H. 2021; Shikonin alleviates endothelial cell injury induced by ox-LDL via AMPK/Nrf2/HO-1 signaling pathway. Evid Based Complement Alternat Med. 2021:5881321. DOI: 10.1155/2021/5881321. PMID: 34912465. PMCID: PMC8668324.
Article17. Liu C, He L, Wang J, Wang Q, Sun C, Li Y, Jia K, Wang J, Xu T, Ming R, Wang Q, Lin N. 2020; Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J Ethnopharmacol. 260:113039. DOI: 10.1016/j.jep.2020.113039. PMID: 32497675.
Article18. Wang J, Chen Z, Feng X, Yin L. 2021; Shikonin ameliorates injury and inflammatory response of LPS-stimulated WI-38 cells via modulating the miR-489-3p/MAP2K1 axis. Environ Toxicol. 36:1775–1784. DOI: 10.1002/tox.23298. PMID: 34089293.
Article19. Ma X, Zou J, He L, Zhang Y. 2014; Dry eye management in a Sjögren's syndrome mouse model by inhibition of p38-MAPK pathway. Diagn Pathol. 9:5. DOI: 10.1186/1746-1596-9-5. PMID: 24443980. PMCID: PMC3976089.
Article20. Ren Y, Cui G, Gao Y. 2021; Research progress on inflammatory mechanism of primary Sjögren syndrome. Zhejiang Da Xue Xue Bao Yi Xue Ban. 50:783–794. DOI: 10.3724/zdxbyxb-2021-0072. PMID: 35347914. PMCID: PMC8931614.21. Chen X, Zhang P, Liu Q, Zhang Q, Gu F, Xu S, Körner H, Wu H, Wei W. 2020; Alleviating effect of paeoniflorin-6'-O-benzene sulfonate in antigen-induced experimental Sjögren's syndrome by modulating B lymphocyte migration via CXCR5-GRK2-ERK/p38 signaling pathway. Int Immunopharmacol. 80:106199. DOI: 10.1016/j.intimp.2020.106199. PMID: 31955068.
Article22. Lin R, Hao D, Dong Y, Wang Y. 2020; Catalpol ameliorates Sjögren's syndrome by modulating interplay of T and B cells. Biomed Pharmacother. 123:109806. DOI: 10.1016/j.biopha.2019.109806. PMID: 31951976.
Article23. Wu M, Yu S, Chen Y, Meng W, Chen H, He J, Shen J, Lin X. 2022; Acteoside promotes B cell-derived IL-10 production and ameliorates autoimmunity. J Leukoc Biol. 112:875–885. DOI: 10.1002/JLB.3MA0422-510R. PMID: 35638582.
Article24. Guo Y, Ji W, Lu Y, Wang Y. 2021; Triptolide reduces salivary gland damage in a non-obese diabetic mice model of Sjögren's syndrome via JAK/STAT and NF-κB signaling pathways. J Clin Biochem Nutr. 68:131–138. DOI: 10.3164/jcbn.20-15. PMID: 33879964. PMCID: PMC8046007.
Article25. Wiench B, Eichhorn T, Paulsen M, Efferth T. 2012; Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. Evid Based Complement Alternat Med. 2012:726025. DOI: 10.1155/2012/726025. PMID: 23118796. PMCID: PMC3478753.
Article26. Bai GZ, Yu HT, Ni YF, Li XF, Zhang ZP, Su K, Lei J, Liu BY, Ke CK, Zhong DX, Wang YJ, Zhao JB. 2013; Shikonin attenuates lipopolysaccharide-induced acute lung injury in mice. J Surg Res. 182:303–311. DOI: 10.1016/j.jss.2012.10.039. PMID: 23158409.
Article27. Wang Z, Liu T, Gan L, Wang T, Yuan X, Zhang B, Chen H, Zheng Q. 2010; Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur J Pharmacol. 643:211–217. DOI: 10.1016/j.ejphar.2010.06.027. PMID: 20599918.
Article28. Lai JL, Liu YH, Liu C, Qi MP, Liu RN, Zhu XF, Zhou QG, Chen YY, Guo AZ, Hu CM. 2017; Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation. 40:1–12. DOI: 10.1007/s10753-016-0447-7. PMID: 27718095.
Article29. Li D, Ren W, Jiang Z, Zhu L. 2018; Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep. 18:4399–4409. DOI: 10.3892/mmr.2018.9427.
Article30. Yu X, Quan J, Long W, Chen H, Wang R, Guo J, Lin X, Mai S. 2018; LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res. 372:178–187. DOI: 10.1016/j.yexcr.2018.09.024. PMID: 30287143.
Article31. Li X, Kang J, Lv H, Liu R, Chen J, Zhang Y, Zhang Y, Yu G, Zhang X, Ning B. 2021; CircPrkcsh, a circular RNA, contributes to the polarization of microglia towards the M1 phenotype induced by spinal cord injury and acts via the JNK/p38 MAPK pathway. FASEB J. 35:e22014. DOI: 10.1096/fj.202100993R.
Article32. Cao N, Shi H, Chen C, Zheng L, Yu C. 2021; Inhibition of the TLR9-dependent p38 MAPK signaling pathway improves the pathogenesis of primary Sjögren's syndrome in the NOD/Ltj mouse. J Biol Regul Homeost Agents. 35:1103–1108. DOI: 10.23812/21-154-L. PMID: 34034463.33. Ye L, Shi H, Yu C, Fu J, Chen C, Wu S, Zhan T, Wang B, Zheng L. 2020; LncRNA Neat1 positively regulates MAPK signaling and is involved in the pathogenesis of Sjögren's syndrome. Int Immunopharmacol. 88:106992. DOI: 10.1016/j.intimp.2020.106992. PMID: 33182021.
Article34. Wei L, Xiong H, Li W, Li B, Cheng Y. 2018; Upregulation of IL-6 expression in human salivary gland cell line by IL-17 via activation of p38 MAPK, ERK, PI3K/Akt, and NF-κB pathways. J Oral Pathol Med. 47:847–855. DOI: 10.1111/jop.12765. PMID: 30007092.
Article35. Fu J, Shi H, Cao N, Wu S, Zhan T, Xie L, Wang Z, Ye L, Li C, Shen Y, Yu C, Zheng L. 2019; Toll-like receptor 9 signaling promotes autophagy and apoptosis via divergent functions of the p38/JNK pathway in human salivary gland cells. Exp Cell Res. 375:51–59. DOI: 10.1016/j.yexcr.2018.12.027. PMID: 30610847.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Multiple Sialolithiasis in the Parotid Gland with Sjogren's Syndrome
- Role of Homeostatic Changes in Salivary Gland Acinar Cells in Primary Sjöögren's Syndrome: A Review
- A Case of Sjogren Syndrome in Parotid Gland
- Ultrasonography of the salivary glands in primary Sjogren's syndrome with positive anti Ro/SS-A antibody
- Ocular Surface Staining Score and Salivary Gland Scintigraphy in Patients with Primary Sjögren’s Syndrome