J Korean Med Sci.
2023 Jun;38(23):e195. 10.3346/jkms.2023.38.e195.
Two Years of Experience and Methodology of Korean COVID-19 Living Clinical Practice Guideline Development
- Affiliations
-
- 1Division of Healthcare Technology Assessment Research, National Evidence-based Healthcare Collaborating Agency, Seoul, Korea
- 2Department of Medical Information, College of Nursing and Health, Kongju National University, Gongju, Korea
- 3Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, National Medical Center, Seoul, Korea
- 5Department of Pulmonary, Allergy and Critical Care Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea
- 6Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
- 7Department of Radiology, College of Medicine, Chungbuk National University, Cheongju, Korea
- 8Department of Laboratory Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 9Department of Laboratory Medicine, Seoul National University-Seoul Metropolitan Government Boramae Hospital, Seoul, Korea
- 10Department of Emergency Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 11Division of Infectious Diseases, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 12Department of Thoracic and Cardiovascular Surgery, Chonnam National University Hospital and Medical School, Gwangju, Korea
- 13Department of Pediatrics, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 14Department of Radiology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 15Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- 16Department of Emergency Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 17Department of Radiology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 18Department of Respiratory and Critical Care Medicine, Inje University Seoul Paik Hospital, Seoul, Korea
- KMID: 2543883
- DOI: http://doi.org/10.3346/jkms.2023.38.e195
Abstract
- Background
In Korea, during the early phase of the coronavirus disease 2019 (COVID-19) pandemic, we responded to the uncertainty of treatments under various conditions, consistently playing catch up with the speed of evidence updates. Therefore, there was high demand for national-level evidence-based clinical practice guidelines for clinicians in a timely manner. We developed evidence-based and updated living recommendations for clinicians through a transparent development process and multidisciplinary expert collaboration.
Methods
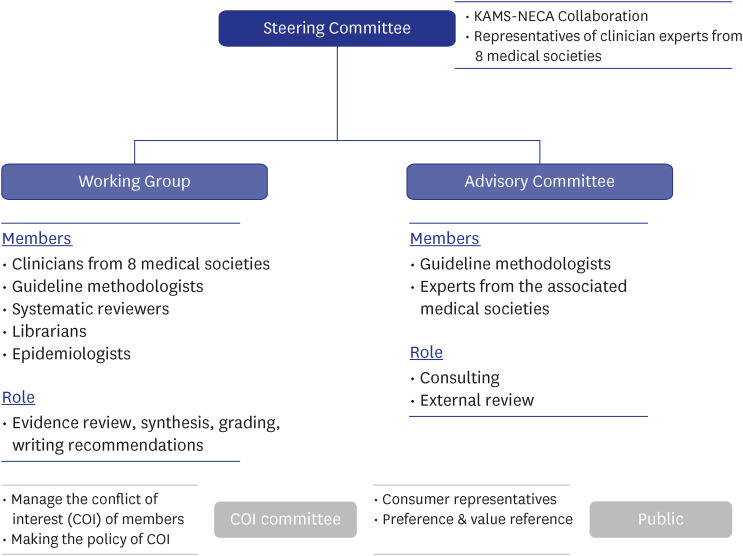
The National Evidence-based Healthcare Collaborating Agency (NECA) and the Korean Academy of Medical Sciences (KAMS) collaborated to develop trustworthy Korean living guidelines. The NECA-supported methodological sections and 8 professional medical societies of the KAMS worked with clinical experts, and 31 clinicians were involved annually. We developed a total of 35 clinical questions, including medications, respiratory/critical care, pediatric care, emergency care, diagnostic tests, and radiological examinations.
Results
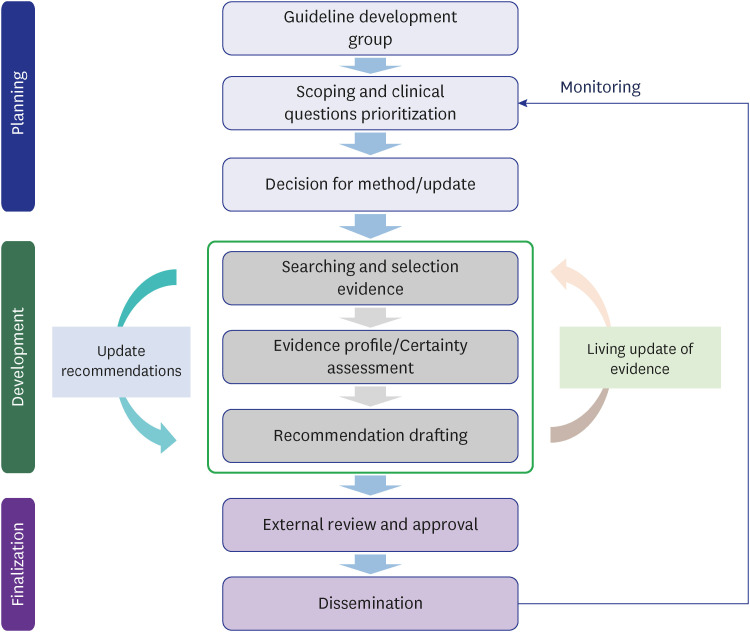
An evidence-based search for treatments began in March 2021 and monthly updates were performed. It was expanded to other areas, and the search interval was organized by a steering committee owing to priority changes. Evidence synthesis and recommendation review was performed by researchers, and living recommendations were updated within 3–4 months.
Conclusion
We provided timely recommendations on living schemes and disseminated them to the public, policymakers and various stakeholders using webpages and social media. Although the output was successful, there were some limitations. The rigor of development issues, urgent timelines for public dissemination, education for new developers, and spread of several new COVID-19 variants have worked as barriers. Therefore, we must prepare systematic processes and funding for future pandemics.
Figure
Reference
-
1. Cheyne S, Fraile Navarro D, Hill K, McDonald S, Tunnicliffe D, White H, et al. Methods for living guidelines: early guidance based on practical experience. J Clin Epidemiol. 2023; 155:84–96. PMID: 36639038.
Article2. Djulbegovic B, Guyatt G. Evidence-based medicine in times of crisis. J Clin Epidemiol. 2020; 126:164–166. PMID: 32659364.
Article3. Kim SB, Ryoo S, Huh K, Joo EJ, Kim YJ, Choi WS, et al. Revised Korean Society of Infectious Diseases/National Evidence-based Healthcarea Collaborating Agency guidelines on the treatment of patients with COVID-19. Infect Chemother. 2021; 53(1):166–219. PMID: 34409790.
Article4. Kowalski SC, Morgan RL, Falavigna M, Florez ID, Etxeandia-Ikobaltzeta I, Wiercioch W, et al. Development of rapid guidelines: 1. systematic survey of current practices and methods. Health Res Policy Syst. 2018; 16(1):61. PMID: 30005712.
Article5. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate COVID-19. N Engl J Med. 2020; 383(18):1757–1766. PMID: 32329974.
Article6. Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. framing the question and deciding on important outcomes. J Clin Epidemiol. 2011; 64(4):395–400. PMID: 21194891.
Article7. Veritas Health Innovation. Covidence systematic review software. Updated 2020. Accessed June 20, 2020. www.covidence.org .8. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. PMID: 22008217.
Article9. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66(4):408–414. PMID: 23337781.
Article10. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155(8):529–536. PMID: 22007046.
Article11. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing;2020.12. National Evidence-based Healthcare Collaborating Agency (NECA)-Korean Academy of Medical Sciences (KAMS) Korean COVID-19 Living Guideline Development Group. COVID-19 Living Guideline. Updated 2022. Accessed June 1, 2022. https://www.neca.re.kr/lay1/bbs/S1T11C174/F/58/list.do .13. Ryoo S, Koh DH, Yu SY, Choi M, Huh K, Yeom JS, et al. Clinical efficacy and safety of interferon (type I and type III) therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2023; 18(3):e0272826. PMID: 36989209.
Article14. Yu SY, Koh DH, Choi M, Ryoo S, Huh K, Yeom JS, et al. Clinical efficacy and safety of interleukin-6 receptor antagonists (tocilizumab and sarilumab) in patients with COVID-19: a systematic review and meta-analysis. Emerg Microbes Infect. 2022; 11(1):1154–1165. PMID: 35343397.
Article15. Kim J, Sung H, Lee H, Kim JS, Shin S, Jeong S, et al. Clinical performance of rapid and point-of-care antigen tests for SARS-CoV-2 variants of concern: a living systematic review and meta-analysis. Viruses. 2022; 14(7):1479. PMID: 35891461.
Article16. Lee HJ, Kim J, Choi M, Choi WI, Joh J, Park J, et al. Early intubation and clinical outcomes in patients with severe COVID-19: a systematic review and meta-analysis. Eur J Med Res. 2022; 27(1):226. PMID: 36329482.
Article17. Lee HJ, Kim J, Choi M, Choi WI, Joh J, Park J, et al. Efficacy and safety of prone position in COVID-19 patients with respiratory failure: a systematic review and meta-analysis. Eur J Med Res. 2022; 27(1):310. PMID: 36572946.
Article18. Choi M, Kim SY, Lee YK. Executive Committee for Clinical Practice Guidelines, The Korean Academy of Medical Sciences. Current status of clinical practice guidelines in Korea. J Korean Med Sci. 2021; 36(6):e35. PMID: 33559406.
Article19. Akl EA, Meerpohl JJ, Elliott J, Kahale LA, Schünemann HJ. Living Systematic Review Network. Living systematic reviews: 4. living guideline recommendations. J Clin Epidemiol. 2017; 91:47–53. PMID: 28911999.20. Hill K, English C, Campbell BC, McDonald S, Pattuwage L, Bates P, et al. Feasibility of national living guideline methods: The Australian Stroke Guidelines. J Clin Epidemiol. 2022; 142:184–193. PMID: 34785347.
Article21. Hewitt J, McDonald S, Poole A, White H, Turner S, Turner T. Weekly updating of guideline recommendations was feasible: the Australian National COVID-19 clinical evidence taskforce. J Clin Epidemiol. 2023; 155:131–136. PMID: 36813003.
Article22. National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing COVID-19. Updated 2021. Accessed April 24, 2023. https://www.nice.org.uk/guidance/ng191/chapter/Update-information .23. MAGIC Evidence Ecosystem Foundation. MAGICapp. Updated 2021. Accessed February 4, 2021. https://app.magicapp.org/#/guidelines .24. Evidence Prime. e-COVID-19 RecMap platform-COVID19 recommendations. Updated 2022. Accessed June 8, 2022. https://covid19.recmap.org/ .25. Lamontagne F, Stegemann M, Agarwal A, Agoritsas T, Siemieniuk R, Rochwerg B, et al. A living WHO guideline on drugs to prevent covid-19. BMJ. 2021; 372(526):n526. PMID: 33649077.26. Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for COVID-19. BMJ. 2020; 370:m3379. PMID: 32887691.27. Yamakawa K, Yamamoto R, Terayama T, Hashimoto H, Ishihara T, Ishimaru G, et al. Japanese rapid/living recommendations on drug management for COVID-19: updated guidelines (September 2021). Acute Med Surg. 2021; 8(1):e706. PMID: 34815889.28. Yamakawa K, Yamamoto R, Terayama T, Hashimoto H, Ishihara T, Ishimaru G, et al. Japanese rapid/living recommendations on drug management for COVID-19: updated guidelines (July 2022). Acute Med Surg. 2022; 9(1):e789. PMID: 36267628.29. Pottie K, Smith M, Matthews M, Santesso N, Magwood O, Kredo T, et al. A multistakeholder development process to prioritize and translate COVID-19 health recommendations for patients, caregivers and the public. A case study of the COVID-19 recommendation map. J Clin Epidemiol. 2022; 148:104–114. PMID: 35500815.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advance in Clinical Practice Guideline Development Methodology
- Clinical Practice Experience of Nursing Students During the COVID-19 Pandemic
- A Comparison of the Perception of and Adherence to the COVID-19 Social Distancing Behavior Guidelines among Health Care Workers, Patients, and General Public
- Factors Associated with Depression in Older Adults Living Alone during the COVID-19 Pandemic
- Types of perception toward non-face-to-face clinical practice among nursing students