J Korean Med Sci.
2023 Jun;38(23):e174. 10.3346/jkms.2023.38.e174.
Real-World Treatment Intensity and Patterns in Patients With Myopic Choroidal Neovascularization: Common Data Model in Ophthalmology
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Seoul National University, Seoul, Korea
- 2Department of Ophthalmology, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Ophthalmology, Seoul National University Hospital, Seoul, Korea
- KMID: 2543845
- DOI: http://doi.org/10.3346/jkms.2023.38.e174
Abstract
- Background
A paucity of data addressing real-world treatment of myopic choroidal neovascularization (mCNV) in the era of anti-vascular endothelial growth factor (VEGF) drugs led us to investigate real-world treatment intensity and treatment patterns in patients with mCNV.
Methods
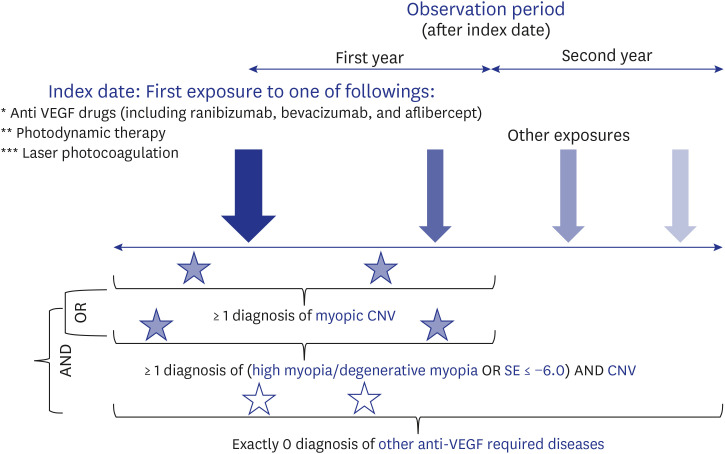
This is a retrospective, observational study using the Observational Medical Outcomes Partnership-Common Data Model database of treatment-naïve patients with mCNV over the 18-year study period (2003–2020). Outcomes were treatment intensity (time trends of total/average number of prescriptions, mean number of prescriptions in the first year and the second year after initiating treatment, proportion of patients with no treatment in the second year) and treatment patterns (subsequent patterns of treatment according to the initial treatment).
Results
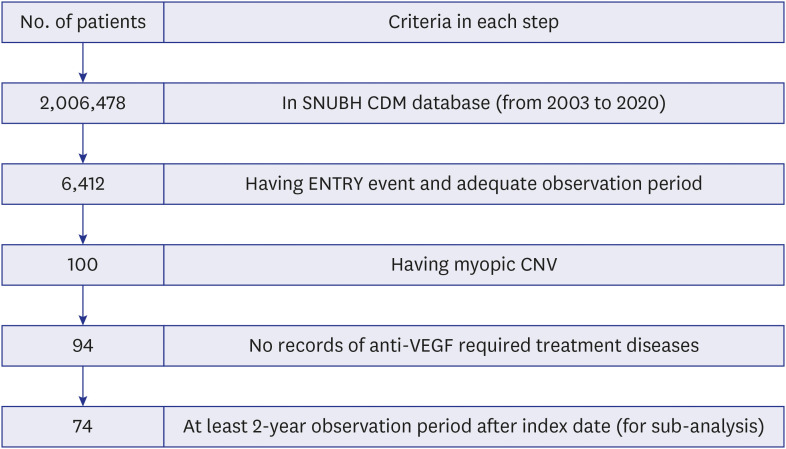
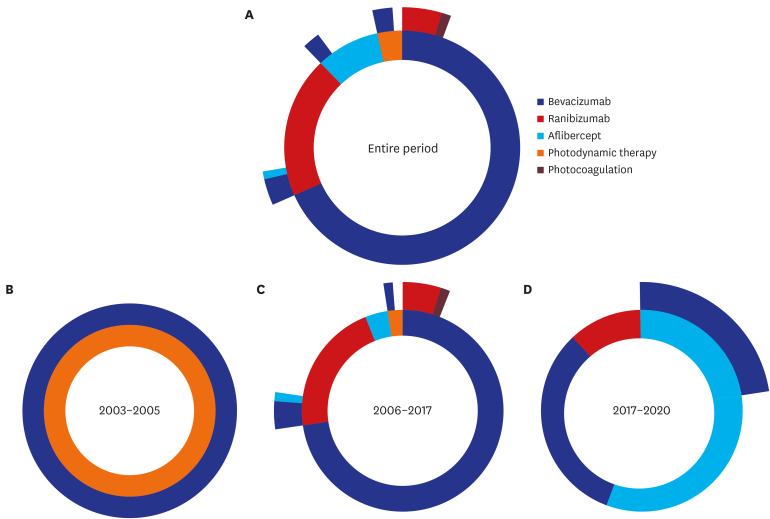
Our final cohort included 94 patients with at-least 1-year observation period. Overall, 96.8% of patients received anti-VEGF drugs as first-line treatment, with most of injections from bevacizumab. The number of anti-VEGF injections in each calendar year showed an increasing trend over time; however, there was a drop in the mean number of injections in the second year compared to the first year from 2.09 to 0.47. About 77% of patients did not receive any treatment in their second year of treatment regardless of drugs. Most of patients (86.2%) followed non-switching monotherapy only and bevacizumab was the most popular choice either in the first-line (68.1%) or in the second-line (53.8%) of treatment. Aflibercept was increasingly used as the first-line treatment for patients with mCNV.
Conclusion
Anti-VEGF drugs have become the treatment of choice and second-line treatment for mCNV over the past decade. Anti-VEGF drugs are effective for the treatment of mCNV as the non-switching monotherapy is the main treatment regimen in most cases and the number of treatments decreases significantly in the second year of treatment.
Keyword
Figure
Reference
-
1. Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012; 32(1):3–16. PMID: 22150586.
Article2. Rim TH, Kim SH, Lim KH, Choi M, Kim HY, Baek SH, et al. Refractive errors in Koreans: the Korea National Health and Nutrition Examination Survey 2008–2012. Korean J Ophthalmol. 2016; 30(3):214–224. PMID: 27247521.
Article3. Kuehn BM. Increase in myopia reported among children during COVID-19 Lockdown. JAMA. 2021; 326(11):999.
Article4. Ma M, Xiong S, Zhao S, Zheng Z, Sun T, Li C. COVID-19 home quarantine accelerated the progression of myopia in children aged 7 to 12 years in China. Invest Ophthalmol Vis Sci. 2021; 62(10):37.
Article5. Neelam K, Cheung CM, Ohno-Matsui K, Lai TY, Wong TY. Choroidal neovascularization in pathological myopia. Prog Retin Eye Res. 2012; 31(5):495–525. PMID: 22569156.
Article6. Ang M, Wong TY. Updates on Myopia: A Clinical Perspective. Singapore, Singapore: Springer Nature;2020.7. Toto L, Di Antonio L, Costantino O, Mastropasqua R. Anti-VEGF therapy in myopic CNV. Curr Drug Targets. 2021; 22(9):1054–1063. PMID: 33511955.
Article8. Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ. Etiology of choroidal neovascularization in young patients. Ophthalmology. 1996; 103(8):1241–1244. PMID: 8764794.
Article9. Miller DG, Singerman LJ. Vision loss in younger patients: a review of choroidal neovascularization. Optom Vis Sci. 2006; 83(5):316–325. PMID: 16699445.
Article10. Stuart A, Ford JA, Duckworth S, Jones C, Pereira A. Anti-VEGF therapies in the treatment of choroidal neovascularisation secondary to non-age-related macular degeneration: a systematic review. BMJ Open. 2015; 5(4):e007746.
Article11. Cohen SY. Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina. 2009; 29(8):1062–1066. PMID: 19734760.
Article12. Wolf S, Balciuniene VJ, Laganovska G, Menchini U, Ohno-Matsui K, Sharma T, et al. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology. 2014; 121(3):682–92.e2. PMID: 24326106.
Article13. Ikuno Y, Ohno-Matsui K, Wong TY, Korobelnik JF, Vitti R, Li T, et al. Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology. 2015; 122(6):1220–1227. PMID: 25745875.
Article14. Willis J, Morse L, Vitale S, Parke DW 2nd, Rich WL, Lum F, et al. Treatment patterns for myopic choroidal neovascularization in the United States: analysis of the IRIS registry. Ophthalmology. 2017; 124(7):935–943. PMID: 28372860.
Article15. Yang MC, Chen YP, Tan EC, Leteneux C, Chang E, Chu CH, et al. Epidemiology, treatment pattern and health care utilization of myopic choroidal neovascularization: a population based study. Jpn J Ophthalmol. 2017; 61(2):159–168. PMID: 28062929.
Article16. Ohno-Matsui K, Suzaki M, Teshima R, Okami N. Real-world data on ranibizumab for myopic choroidal neovascularization due to pathologic myopia: results from a post-marketing surveillance in Japan. Eye (Lond). 2018; 32(12):1871–1878. PMID: 30158574.
Article17. Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. 2017; 101(3):353–359. PMID: 27215744.
Article18. Wu TT, Kung YH. Two-year outcome of intravitreal injections of ranibizumab for myopic choroidal neovascularization. J Ocul Pharmacol Ther. 2014; 30(10):837–841. PMID: 25162313.
Article19. Park SJ, Kwon KE, Choi NK, Park KH, Woo SJ. Prevalence and incidence of exudative age-related macular degeneration in South Korea: a nationwide population-based study. Ophthalmology. 2015; 122(10):2063–70.e1. PMID: 26208437.
Article20. Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform. 2015; 216:574–578. PMID: 26262116.21. Lee KA, Jin HY, Kim YJ, Im YJ, Kim EY, Park TS. Treatment patterns of type 2 diabetes assessed using a common data model based on electronic health records of 2000–2019. J Korean Med Sci. 2021; 36(36):e230. PMID: 34519186.
Article22. Mun Y, You SC, Lee DY, Kim S, Chung YR, Lee K, et al. Real-world incidence of endopthalmitis after intravitreal anti-VEGF injections in Korea: findings from the Common Data Model in ophthalmology. Epidemiol Health. 2021; 43:e2021097. PMID: 34773936.
Article23. Mun Y, Park C, Lee DY, Kim TM, Jin KW, Kim S, et al. Real-world treatment intensities and pathways of macular edema following retinal vein occlusion in Korea from Common Data Model in ophthalmology. Sci Rep. 2022; 12(1):10162. PMID: 35715561.
Article24. Kang DY, Kim H, Ko S, Kim H, Shinn J, Kang MG, et al. Sodium-glucose cotransporter-2 inhibitor-related diabetic ketoacidosis: accuracy verification of operational definition. J Korean Med Sci. 2022; 37(7):e53. PMID: 35191230.
Article25. Ohno-Matsui K, Ikuno Y, Lai TY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res. 2018; 63:92–106. PMID: 29111299.
Article26. Tah V, Orlans HO, Hyer J, Casswell E, Din N, Sri Shanmuganathan V, et al. Anti-VEGF therapy and the retina: an update. J Ophthalmol. 2015; 2015:627674. PMID: 26417453.
Article27. Wakabayashi T, Ikuno Y, Gomi F, Hamasaki T, Tano Y. Intravitreal bevacizumab vs sub-tenon triamcinolone acetonide for choroidal neovascularization attributable to pathologic myopia. Am J Ophthalmol. 2009; 148(4):591–596.e1. PMID: 19589497.
Article28. Karasu B, Celebi AR. The efficacy of different anti-vascular endothelial growth factor agents and prognostic biomarkers in monitoring of the treatment for myopic choroidal neovascularization. Int Ophthalmol. 2022; 42(9):2729–2740. PMID: 35357641.
Article29. Mallone F, Giustolisi R, Franzone F, Marenco M, Plateroti R, Nebbioso M, et al. Ten-year outcomes of intravitreal bevacizumab for myopic choroidal neovascularization: analysis of prognostic factors. Pharmaceuticals (Basel). 2021; 14(10):1042. PMID: 34681267.
Article30. Hamilton RD, Clemens A, Minnella AM, Lai TY, Dai H, Sakamoto T, et al. Real-world effectiveness and safety of ranibizumab for the treatment of myopic choroidal neovascularization: Results from the LUMINOUS study. PLoS One. 2020; 15(1):e0227557. PMID: 31961888.
Article31. Tan NW, Ohno-Matsui K, Koh HJ, Nagai Y, Pedros M, Freitas RL, et al. Long-term outcomes of ranibizumab treatment of myopic choroidal neovascularization in East-Asian patients from the RADIANCE study. Retina. 2018; 38(11):2228–2238. PMID: 28961671.
Article32. Chen Y, Sharma T, Li X, Song Y, Chang Q, Lin R, et al. Ranibizumab versus verteporfin photodynamic therapy in Asian patients with myopic choroidal neovascularization: BRILLIANCE, a 12-month, randomized, double-masked study. Retina. 2019; 39(10):1985–1994. PMID: 30204730.
Article33. Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014; 98(9):1144–1167. PMID: 25136079.
Article34. Amoaku WM, Ghanchi F, Bailey C, Banerjee S, Banerjee S, Downey L, et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye (Lond). 2020; 34(Suppl 1):1–51.
Article35. Erden B, Bölükbaşı S, Baş E, Çakır A. Comparison of intravitreal aflibercept and ranibizumab for treatment of myopic choroidal neovascularization: one-year results-a retrospective, comparative study. J Ophthalmol. 2019; 2019:8639243. PMID: 32082619.
Article36. Wang JK, Huang TL, Chang PY, Chen YT, Chang CW, Chen FT, et al. Intravitreal aflibercept versus bevacizumab for treatment of myopic choroidal neovascularization. Sci Rep. 2018; 8(1):14389. PMID: 30258077.
Article37. Wu B, Wu H, Liu X, Lin H, Li J. Ranibizumab versus bevacizumab for ophthalmic diseases related to neovascularisation: a meta-analysis of randomised controlled trials. PLoS One. 2014; 9(7):e101253. PMID: 24983855.
Article38. Munk MR, Rückert R, Zinkernagel M, Ebneter A, Wolf S. The role of anti-VEGF agents in myopic choroidal neovascularization: current standards and future outlook. Expert Opin Biol Ther. 2016; 16(4):477–487. PMID: 26666589.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Therapeutic Effect of Intravitreal Bevacizumab (Avastin) on Myopic Choroidal Neovascularization
- Availability of Optical Coherence Tomography in Diagnosis and Classification of Choroidal Neovascularization
- Choroid in Myopic Choroidal Neovascularization Measured Using SD-OCT
- Therapeutic Effect of Photodynamic Therapy with Indocyanine Green Dye in the Treatment of Choroidal Neovascularization
- Short-term Efficacy of Intravitreal Ranibizumab for Myopic Choroidal Neovascularization