Kosin Med J.
2023 Jun;38(2):126-133. 10.7180/kmj.23.113.
Intraoperative tumor localization using a titanium ring strip in totally laparoscopic distal gastrectomy for middle-third gastric cancer
- Affiliations
-
- 1Department of Surgery, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 2Medical Research Institute, Institute, Pusan National University Hospital, Busan, Korea
- 3Department of Surgery, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Busan, Korea
- KMID: 2543332
- DOI: http://doi.org/10.7180/kmj.23.113
Abstract
- Background
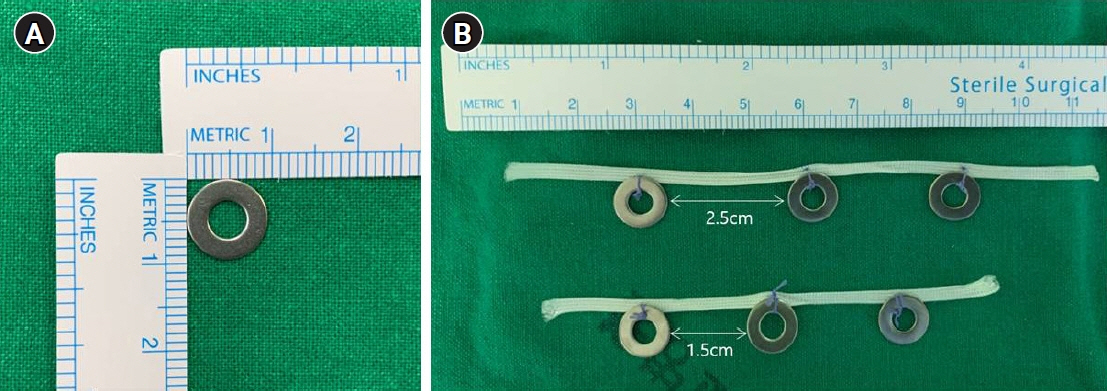
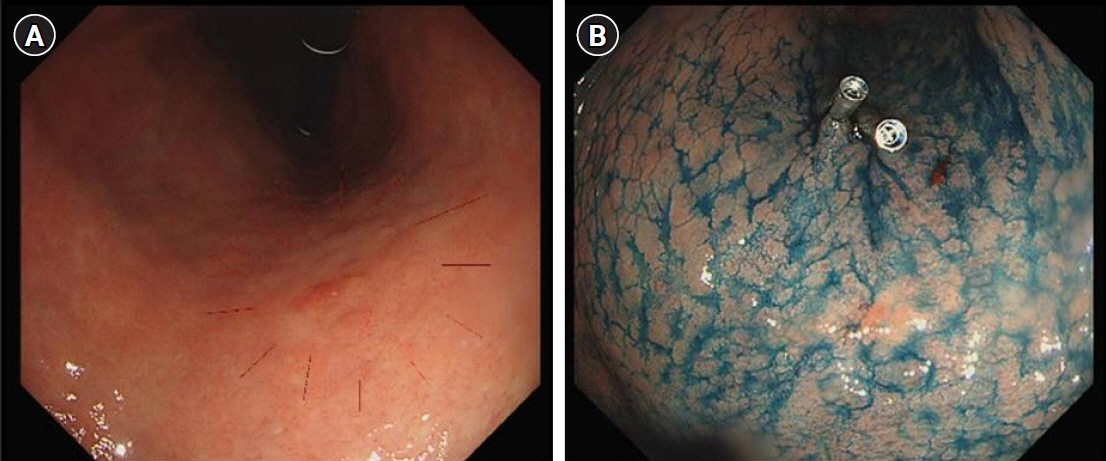
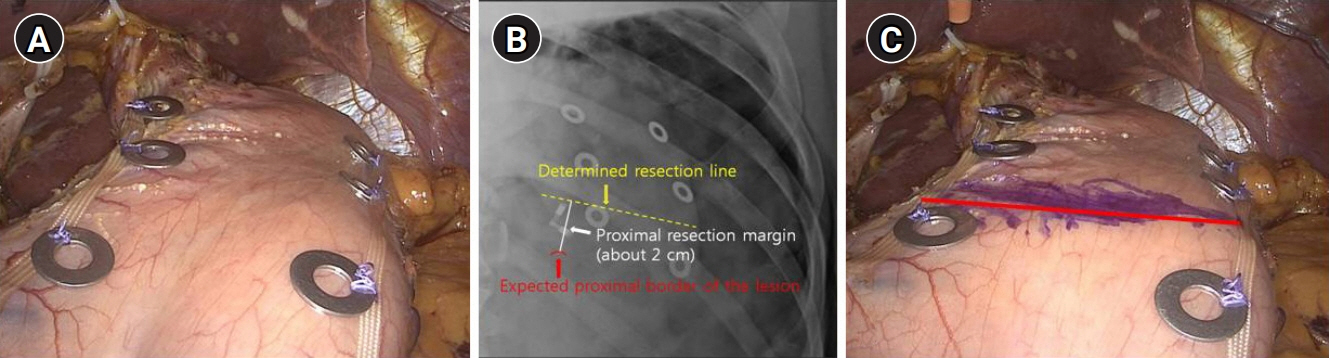
This study presents a novel technical tip for intraoperative tumor localization and determination of the proximal resection line using a titanium ring strip for totally laparoscopic distal gastrectomy in patients with middle-third gastric cancer and describes the short-term results of its application.
Methods
In total, 42 patients with middle-third gastric cancer who underwent intraoperative tumor localization using a titanium ring strip and determination of the proximal resection line through intraoperative radiography between January 2020 and December 2021 were enrolled in this study. We retrospectively analyzed patients’ prospectively collected clinical, pathological, and surgical data.
Results
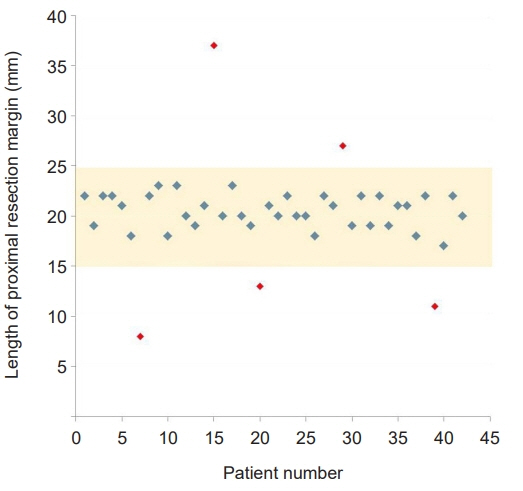
Twenty-six men and 16 women with a mean age of 58.3±12.5 years were enrolled. The mean operation time and estimated blood loss were 212.6±43.0 minutes and 122.4±77.6 mL, respectively. The lengths of the proximal and distal resection margin were 2.0±0.4 cm (range, 0.8–3.7 cm) and 10.5±4.1 cm (range, 0.4–20.4 cm), respectively. Roux-en-Y anastomosis was performed in 30 patients, while Billroth II with Braun anastomosis was performed in 12 patients. There were no procedure-related complications, and the mean postoperative hospital stay was 7.2±1.9 days. For all patients, the negative proximal resection margin was confirmed by postoperative pathological examinations.
Conclusions
Intraoperative tumor localization and determination of the proximal resection line using a titanium ring strip is a useful alternative method that can be easily and safely performed. This method is especially useful for patients with middle-third gastric cancer requiring an appropriate proximal resection margin.
Keyword
Figure
Reference
-
References
1. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014; 14:87–104.2. Kim A, Lee JB, Ko Y, Park T, Jo H, Jang JK, et al. Larger remaining stomach volume is associated with better nutrition and muscle preservation in patients with gastric cancer receiving distal gastrectomy with gastroduodenostomy. J Gastric Cancer. 2022; 22:145–55.3. Heneghan HM, Yimcharoen P, Brethauer SA, Kroh M, Chand B. Influence of pouch and stoma size on weight loss after gastric bypass. Surg Obes Relat Dis. 2012; 8:408–15.4. Braghetto I, Cortes C, Herquinigo D, Csendes P, Rojas A, Mushle M, et al. Evaluation of the radiological gastric capacity and evolution of the BMI 2-3 years after sleeve gastrectomy. Obes Surg. 2009; 19:1262–9.5. Noshiro H, Ohuchida K, Kawamoto M, Ishikawa M, Uchiyama A, Shimizu S, et al. Intraabdominal Roux-en-Y reconstruction with a novel stapling technique after laparoscopic distal gastrectomy. Gastric Cancer. 2009; 12:164–9.6. Takaori K, Nomura E, Mabuchi H, Lee SW, Agui T, Miyamoto Y, et al. A secure technique of intracorporeal Roux-Y reconstruction after laparoscopic distal gastrectomy. Am J Surg. 2005; 189:178–83.7. Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019; 5:506–13.8. Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, et al. Long-term outcomes of laparoscopic distal gastrectomy for locally advanced gastric cancer: the KLASS-02-RCT randomized clinical trial. J Clin Oncol. 2020; 38:3304–13.9. Ahn SH, Son SY, Jung DH, Park DJ, Kim HH. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg. 2014; 219:933–43.10. Kunisaki C, Makino H, Yamaguchi N, Izumisawa Y, Miyamato H, Sato K, et al. Surgical advantages of reduced-port laparoscopic gastrectomy in gastric cancer. Surg Endosc. 2016; 30:5520–8.11. Inaki N, Tsuji T, Doden K, Sakimura Y, Tawara H, Matsui R, et al. Reduced port laparoscopic gastrectomy for gastric cancer. Transl Gastroenterol Hepatol. 2016; 1:38.12. Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg. 2015; 8:1–10.13. Luigiano C, Ferrara F, Morace C, Mangiavillano B, Fabbri C, Cennamo V, et al. Endoscopic tattooing of gastrointestinal and pancreatic lesions. Adv Ther. 2012; 29:864–73.14. Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Song SY, et al. Intraoperative tumor localization using laparoscopic ultrasonography in laparoscopic-assisted gastrectomy. Surg Endosc. 2005; 19:1353–7.15. Park DJ, Lee HJ, Kim SG, Jung HC, Song IS, Lee KU, et al. Intraoperative gastroscopy for gastric surgery. Surg Endosc. 2005; 19:1358–61.16. Kim HI, Hyung WJ, Lee CR, Lim JS, An JY, Cheong JH, et al. Intraoperative portable abdominal radiograph for tumor localization: a simple and accurate method for laparoscopic gastrectomy. Surg Endosc. 2011; 25:958–63.17. Ohdaira T, Nagai H. Intraoperative localization of early-stage upper gastrointestinal tumors using a magnetic marking clip-detecting system. Surg Endosc. 2007; 21:810–5.18. Joo HY, Lee BE, Choi CI, Kim DH, Kim GH, Jeon TY, et al. Tumor localization using radio-frequency identification clip marker: experimental results of an ex vivo porcine model. Surg Endosc. 2019; 33:1441–50.19. Xuan Y, Hur H, Byun CS, Han SU, Cho YK. Efficacy of intraoperative gastroscopy for tumor localization in totally laparoscopic distal gastrectomy for cancer in the middle third of the stomach. Surg Endosc. 2013; 27:4364–70.20. Kojima F, Sato T, Tsunoda S, Takahata H, Hamaji M, Komatsu T, et al. Development of a novel marking system for laparoscopic gastrectomy using endoclips with radio frequency identification tags: feasibility study in a canine model. Surg Endosc. 2014; 28:2752–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracorporeal Anastomosis in Laparoscopic Gastric Cancer Surgery
- A comparison between two methods for tumor localization during totally laparoscopic distal gastrectomy in patients with gastric cancer
- Comparison of Laparoscopy-Assisted and Totally Laparoscopic Distal Gastrectomy: The Short-Term Outcome at a Low Volume Center
- Application of Near-Infrared Fluorescence Imaging with Indocyanine Green in Totally Laparoscopic Distal Gastrectomy
- Comparison of laparoscopy-assisted and totally laparoscopic Billroth-II distal gastrectomy for gastric cancer