Kosin Med J.
2023 Jun;38(2):117-125. 10.7180/kmj.23.103.
The solid predominant subtype as an independent risk factor for recurrence in patients with pathologic stage I lung adenocarcinoma
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Daegu Catholic University School of Medicine, Daegu, Korea
- KMID: 2543331
- DOI: http://doi.org/10.7180/kmj.23.103
Abstract
- Background
Increasingly many patients have been diagnosed with stage I adenocarcinoma due to the use of low-dose chest computed tomography for lung cancer screening. Therefore, this study aimed to analyze tumor recurrence based on the predominant subtype in patients with stage I lung adenocarcinoma who underwent lobectomy.
Methods
We retrospectively analyzed 114 patients who underwent lobectomy for pathologic stage I lung adenocarcinoma from June 2001 to July 2019.
Results
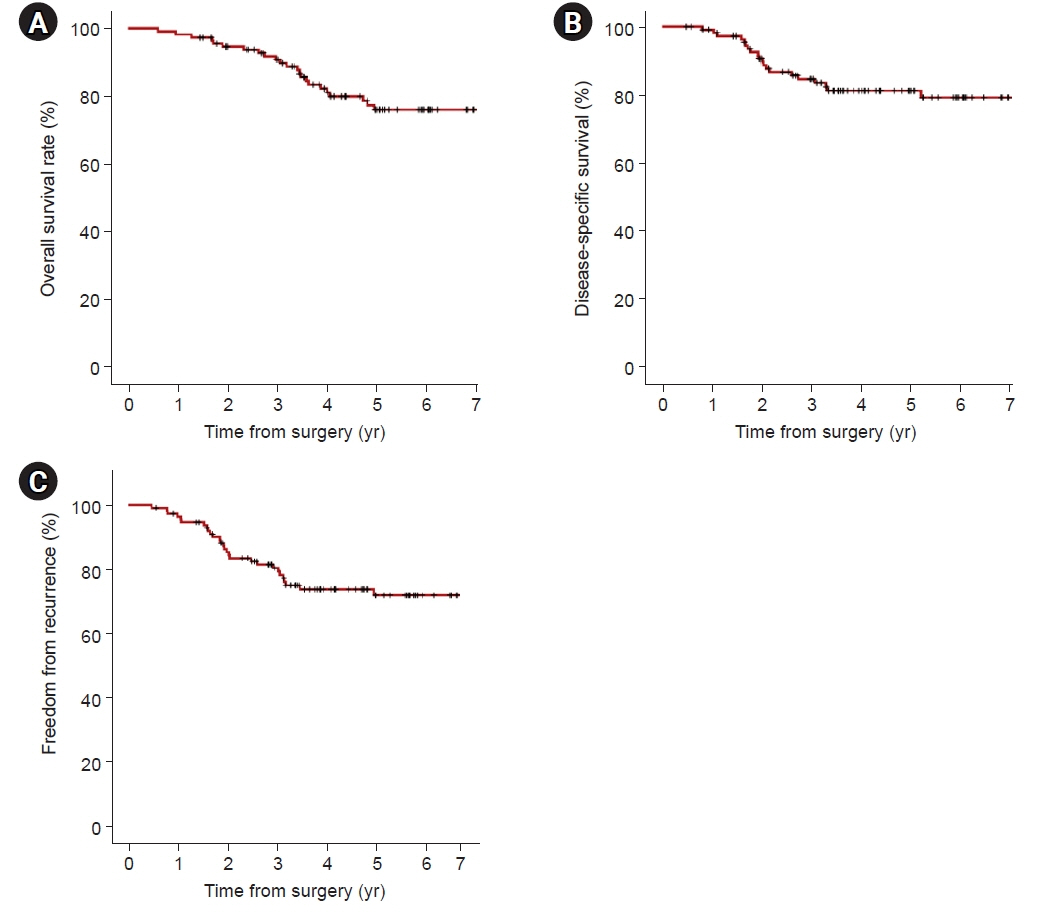
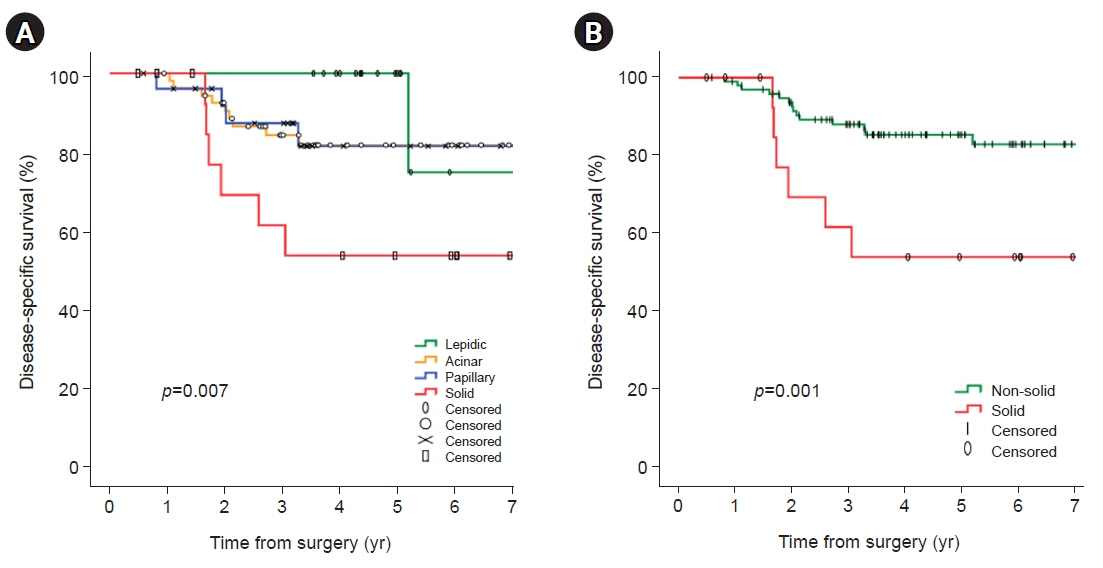
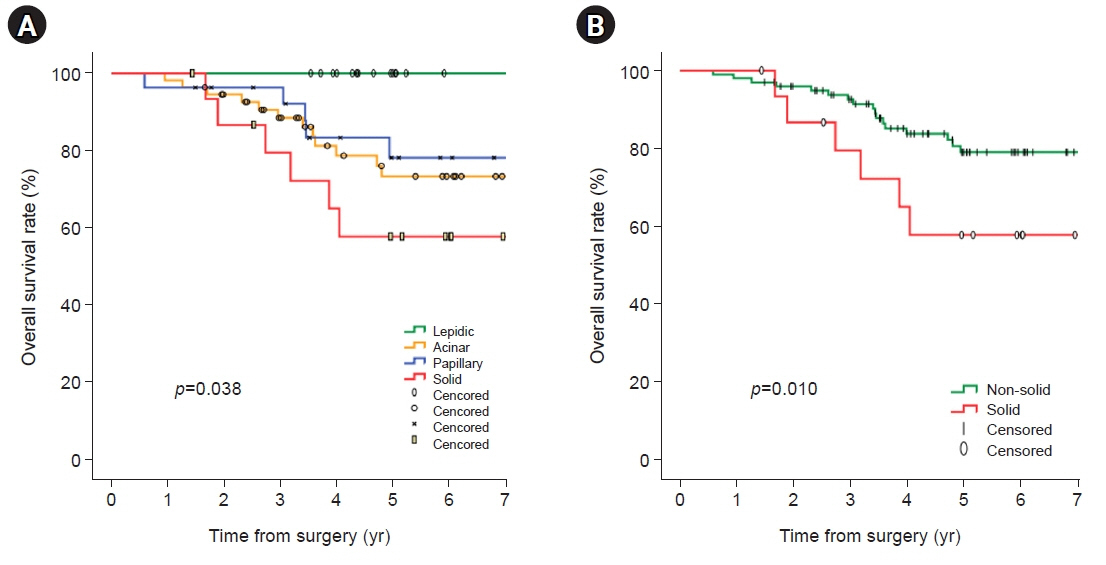
In univariate analyses, significant factors were current smoking at the time of surgery (p=0.029), pathologic tumor size (p=0.006), central tumor location (p=0.003), maximum standardized uptake value on positron emission tomography-computed tomography (p=0.001), and the solid predominant subtype (p=0.012). In the multivariate analysis, only the solid predominant subtype (hazard ratio, 9.702; 95% confidence interval, 1.179–79.874; p=0.035) was an independent risk factor.
Conclusions
If the solid subtype is predominant in pathologic findings, adjuvant chemotherapy after standard surgical resection may be considered to help reduce the risk of tumor recurrence and increase survival.
Figure
Reference
-
References
1. Albano D, Bilfinger T, Nemesure B. 1-, 3-, and 5-year survival among early-stage lung cancer patients treated with lobectomy vs SBRT. Lung Cancer (Auckl). 2018; 9:65–71.2. Shin SH, Jeong DY, Lee KS, Cho JH, Choi YS, Lee K, et al. Which definition of a central tumour is more predictive of occult mediastinal metastasis in nonsmall cell lung cancer patients with radiological N0 disease? Eur Respir J. 2019; 53:1801508.3. Lou F, Huang J, Sima CS, Dycoco J, Rusch V, Bach PB. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013; 145:75–82.4. Wu CF, Fu JY, Yeh CJ, Liu YH, Hsieh MJ, Wu YC, et al. Recurrence risk factors analysis for stage I non-small cell lung cancer. Medicine (Baltimore). 2015; 94:e1337.5. Shiono S, Abiko M, Sato T. Limited resection for clinical Stage IA non-small-cell lung cancers based on a standardized-uptake value index. Eur J Cardiothorac Surg. 2013; 43:e7–12.6. Zhang Y, Sun Y, Xiang J, Zhang Y, Hu H, Chen H. A clinicopathologic prediction model for postoperative recurrence in stage Ia non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014; 148:1193–9.7. Kang SW, Jeong WG, Lee JE, Oh IJ, Song SY, Lee BC, et al. Prognostic significance of location index in resected T1-sized early-stage non-small cell lung cancer. Acta Radiol. 2023; 64:1028–37.8. Huang H, Wang T, Hu B, Pan C. Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg. 2015; 99:1130–9.9. Tsao MS, Marguet S, Le Teuff G, Lantuejoul S, Shepherd FA, Seymour L, et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol. 2015; 33:3439–46.10. Yoshizawa A, Motoi N, Riely GJ, Sima CS, Gerald WL, Kris MG, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011; 24:653–64.11. Motono N, Matsui T, Machida Y, Usuda K, Uramoto H. Prognostic significance of histologic subtype in pStage I lung adenocarcinoma. Med Oncol. 2017; 34:100.12. Hung JJ. Histologic subtype component predicts lymph node micrometastasis and prognosis in patients with stage I lung adenocarcinoma. J Thorac Dis. 2017; 9:3623–5.13. Hung JJ, Yeh YC, Jeng WJ, Wu YC, Chou TY, Hsu WH. Factors predicting occult lymph node metastasis in completely resected lung adenocarcinoma of 3 cm or smaller. Eur J Cardiothorac Surg. 2016; 50:329–36.14. Hung JJ, Yeh YC, Jeng WJ, Wu KJ, Huang BS, Wu YC, et al. Predictive value of the international association for the study of lung cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol. 2014; 32:2357–64.15. Tsutani Y, Miyata Y, Kushitani K, Takeshima Y, Yoshimura M, Okada M. Propensity score-matched analysis of adjuvant chemotherapy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2014; 148:1179–85.16. Park SY, Lee JG, Kim J, Byun GE, Bae MK, Lee CY, et al. Efficacy of platinum-based adjuvant chemotherapy in T2aN0 stage IB non-small cell lung cancer. J Cardiothorac Surg. 2013; 8:151.17. Nagasaka M, Gadgeel SM. Role of chemotherapy and targeted therapy in early-stage non-small cell lung cancer. Expert Rev Anticancer Ther. 2018; 18:63–70.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of Cigarette Smoking in Association with Histologic Subtypes of Resected Lung Adenocarcinoma
- Effect of Adjuvant Chemotherapy after Complete Resection for Pathologic Stage IB Lung Adenocarcinoma in High-Risk Patients as Defined by a New Recurrence Risk Scoring Model
- Clinical Impact of Genomic and Pathway Alterations in Stage I EGFR-Mutant Lung Adenocarcinoma
- Expression of Transforming Growth Factor beta1 and Cadherins in Lung Adenocarcinoma
- Prognostic Significance of Mucinous Histologic Subtype on Oncologic Outcomes in Patients With Colorectal Cancer