Nutr Res Pract.
2023 Jun;17(3):421-437. 10.4162/nrp.2023.17.3.421.
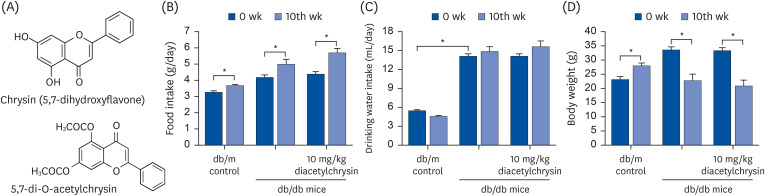
Highly water-soluble diacetyl chrysin ameliorates diabetes-associated renal fibrosis and retinal microvascular abnormality in db/db mice
- Affiliations
-
- 1Department of Food and Nutrition and Nutrition and Korean Institute of Nutrition, Hallym University, Chuncheon 24252, Korea
- 2Department of Pharmacology, College of Medicine, Hallym University, Chuncheon 24252, Korea
- 3FrontBio Inc., Chuncheon 24232, Korea
- 4Department of Biochemistry, College of Medicine, Hallym University, Chuncheon 24252, Korea
- KMID: 2542840
- DOI: http://doi.org/10.4162/nrp.2023.17.3.421
Abstract
- BACKGROUND/OBJECTIVES
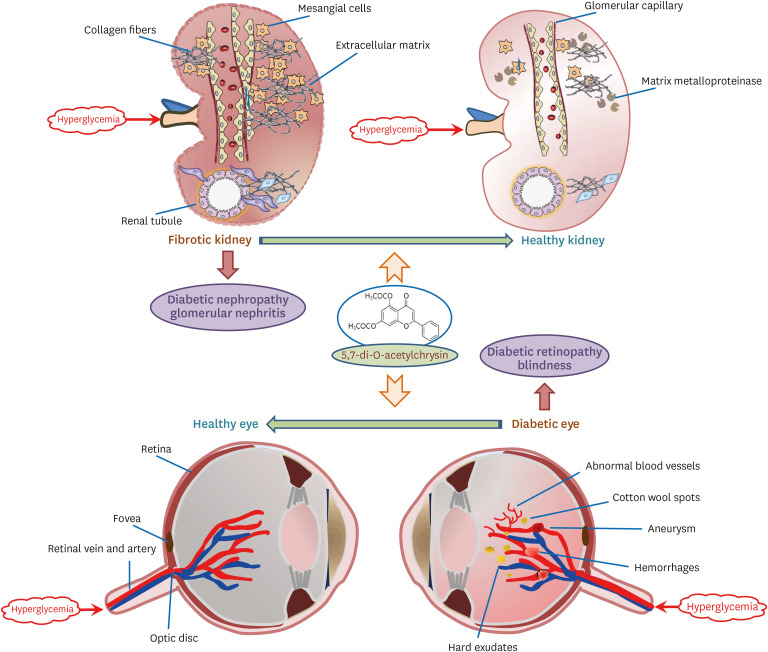
Chronic or intermittent hyperglycemia is associated with the development of diabetic complications. Oxidative stress and inflammation can be altered by hyperglycemia in diverse tissues, including kidneys and eyes, and play a pivotal role in diabetic complications. Our previous studies showed that the water-insoluble 5,7-dihydroxyflvone chrysin effectively combats diabetic damages incurred in diabetic kidneys and retinas. The current study employed the newly-synthesized 5.7-di-O-acetylchrysin, having higher solubility than chrysin, to compare the effects on diabetes-associated renal fibrosis and abnormal retinal neovascularization.
MATERIALS/METHODS
In the in vivo study, db/db mice as animal models of type 2 diabetes were orally administrated 10 mg/kg BW diacetylchrysin, daily for 10 weeks.
RESULTS
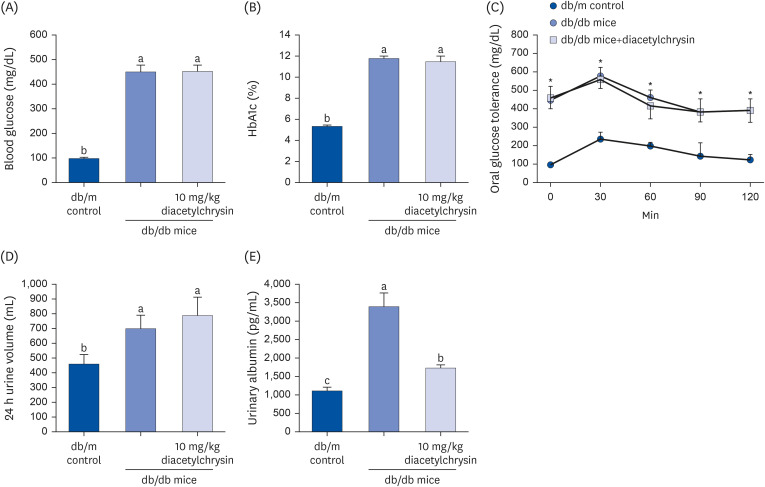
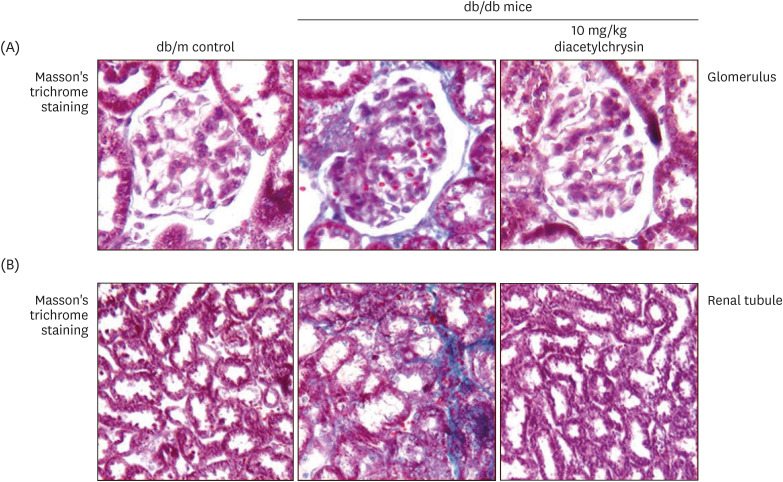
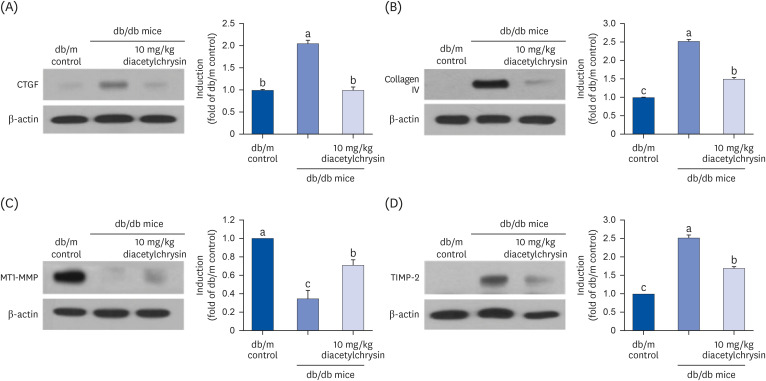
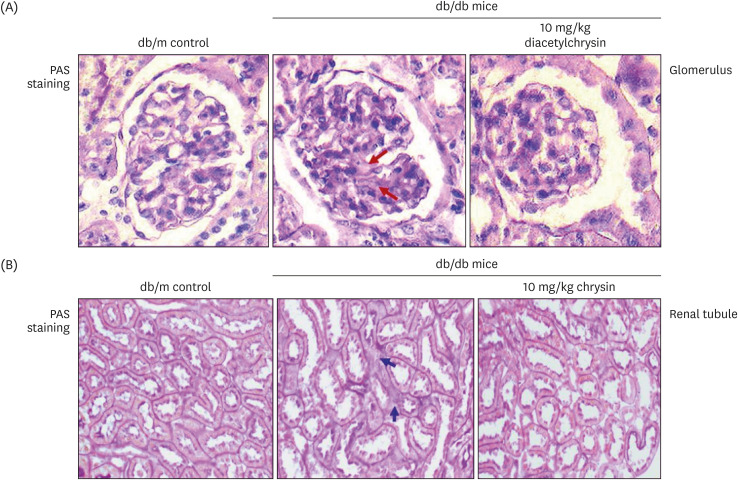
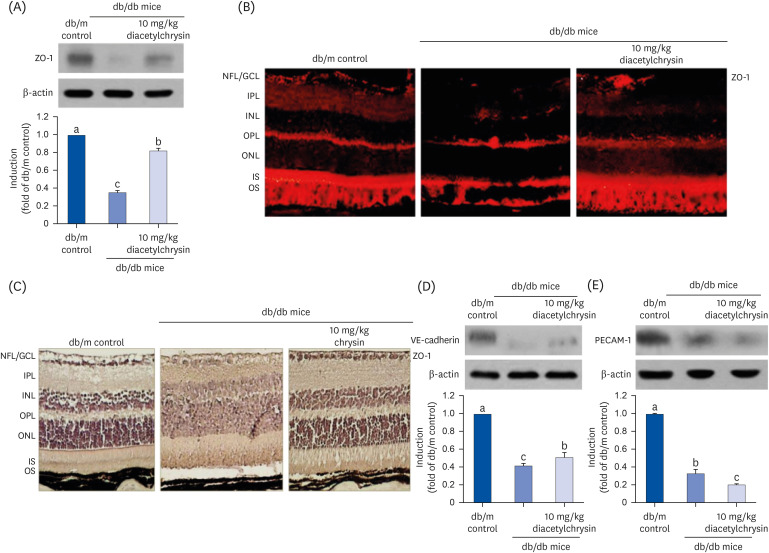
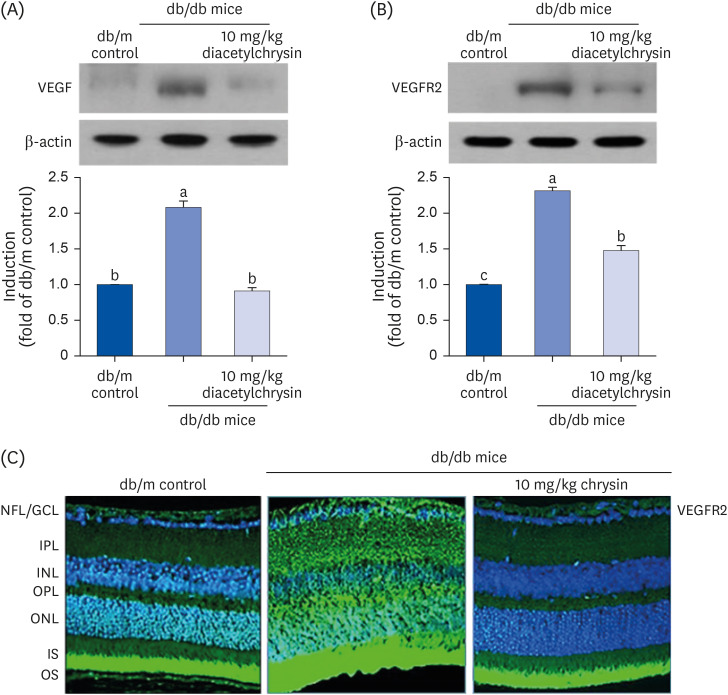
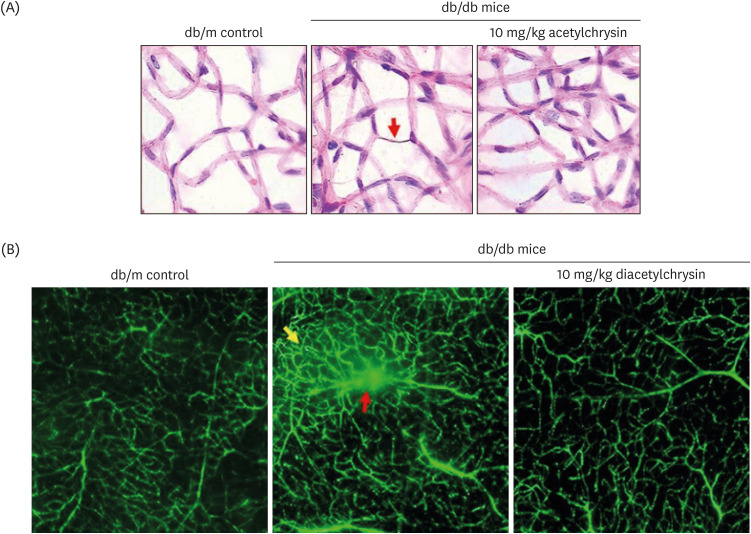
Unlike chrysin, oral administration of 10 mg/kg diacetylchrysin did not lower the blood glucose level and 24 h urine volume in db/db mice. Nevertheless, the urinary albumin excretion was markedly reduced. The administration of diacetylchrysin also diminished the deposition of collagen fibers in diabetic glomeruli and tubules by suppressing the induction of connective tissue growth factor and collagen IV in diabetic kidneys. Supplying diacetylchrysin enhanced the membrane type-1 matrix metalloproteinase (MMP) expression reduced in diabetic kidneys, while the tissue inhibitor of MMP-2 induction was attenuated in diacetylchrysin-challenged diabetic kidneys. In addition, supplementing diacetylchrysin to diabetic mice ameliorated renal injury due to glomerulosclerosis and tubular interstitial fibrosis. Furthermore, the reduced retinal inductions of Zonula occludens-1 and vascular endothelial cadherin in db/db mice were elevated in the retinal tissues of diacetylchrysintreated animals. Oral administration of diacetylchrysin curtailed the induction of vascular endothelial growth factor (VEGF) and VEGF receptor 2 in db/db mice, ultimately retarding diabetes-associated retinal neovascularization. Additionally, the retinal formation of acellular capillaries with leaky vessels was reduced in diacetylchrysin-treated db/db mice.
CONCLUSION
Diacetylchrysin may act as a potent pro-health agent for treating renal fibrosisassociated diabetic nephropathy and retinal neovascularization-associated diabetic retinopathy.
Keyword
Figure
Reference
-
1. Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otín M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009; 11:3071–3109. PMID: 19489690.2. Gilbert MP. Screening and treatment by the primary care provider of common diabetes complications. Med Clin North Am. 2015; 99:201–219. PMID: 25456651.3. Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T. Macrovascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020; 18:110–116. PMID: 30961498.4. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020; 16:377–390. PMID: 32398868.5. Park S, Kang HJ, Jeon JH, Kim MJ, Lee IK. Recent advances in the pathogenesis of microvascular complications in diabetes. Arch Pharm Res. 2019; 42:252–262. PMID: 30771210.6. Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005; 28:164–176. PMID: 15616252.7. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010; 376:124–136. PMID: 20580421.8. Adki KM, Kulkarni YA. Potential biomarkers in diabetic retinopathy. Curr Diabetes Rev. 2020; 16:971–983. PMID: 32065092.9. Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019; 5:41. PMID: 31197153.10. Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007; 27:195–207. PMID: 17418688.11. Menon MC, Chuang PY, He CJ. The glomerular filtration barrier: components and crosstalk. Int J Nephrol. 2012; 2012:749010. PMID: 22934182.12. Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008; 74:22–36. PMID: 18418356.13. Gorriz JL, Martinez-Castelao A. Proteinuria: detection and role in native renal disease progression. Transplant Rev (Orlando). 2012; 26:3–13. PMID: 22137726.14. Kanasaki K, Taduri G, Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol (Lausanne). 2013; 4:7. PMID: 23390421.15. Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011; 7:684–696. PMID: 22009250.16. Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014; 124:2299–2306. PMID: 24892703.17. Arboleda-Velasquez JF, Valdez CN, Marko CK, D’Amore PA. From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr Diab Rep. 2015; 15:573. PMID: 25620405.18. Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016; 2:16012. PMID: 27159554.19. Al-Latayfeh M, Silva PS, Sun JK, Aiello LP. Antiangiogenic therapy for ischemic retinopathies. Cold Spring Harb Perspect Med. 2012; 2:a006411. PMID: 22675660.20. Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015; 49:67–81. PMID: 26113211.21. Boyer DS, Hopkins JJ, Sorof J, Ehrlich JS. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013; 4:151–169. PMID: 24324855.22. Zheng HX, Qi SS, He J, Hu CY, Han H, Jiang H, Li XS. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/Smad expression. J Agric Food Chem. 2020; 68:4399–4410. PMID: 32192334.23. Kang MK, Park SH, Choi YJ, Shin D, Kang YH. Chrysin inhibits diabetic renal tubulointerstitial fibrosis through blocking epithelial to mesenchymal transition. J Mol Med (Berl). 2015; 93:759–772. PMID: 26062793.24. Kang MK, Park SH, Kim YH, Lee EJ, Antika LD, Kim DY, Choi YJ, Kang YH. Dietary compound chrysin inhibits retinal neovascularization with abnormal capillaries in db/db mice. Nutrients. 2016; 8:782. PMID: 27918469.25. Zhou L, Zhang P, Yang G, Lin R, Wang W, Liu T, Zhang L, Zhang J. Solubility of chrysin in ethanol and water mixtures. J Chem Eng Data. 2014; 59:2215–2220.26. Hwang SH, Kim HY, Zuo G, Wang Z, Lee JY, Lim SS. Anti-glycation, carbonyl trapping and anti-inflammatory activities of chrysin derivatives. Molecules. 2018; 23:1752. PMID: 30018253.27. Zhang A, Huang S. Progress in pathogenesis of proteinuria. Int J Nephrol. 2012; 2012:314251. PMID: 22693670.28. Zoja C, Abbate M, Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant. 2015; 30:706–712. PMID: 25087196.29. Capitão M, Soares R. Angiogenesis and inflammation crosstalk in diabetic retinopathy. J Cell Biochem. 2016; 117:2443–2453. PMID: 27128219.30. Kusuhara S, Fukushima Y, Ogura S, Inoue N, Uemura A. Pathophysiology of diabetic retinopathy: the old and the new. Diabetes Metab J. 2018; 42:364–376. PMID: 30362302.31. Bhatti JS, Sehrawat A, Mishra J, Sidhu IS, Navik U, Khullar N, Kumar S, Bhatti GK, Reddy PH. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022; 184:114–134. PMID: 35398495.32. Zheng S, Huang K, Tong T. Efficacy and mechanisms of oleuropein in mitigating diabetes and diabetes complications. J Agric Food Chem. 2021; 69:6145–6155. PMID: 34042426.33. Oliveira S, Monteiro-Alfredo T, Silva S, Matafome P. Curcumin derivatives for type 2 diabetes management and prevention of complications. Arch Pharm Res. 2020; 43:567–581. PMID: 32557163.34. Khan M, Liu H, Wang J, Sun B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: a comprehensive review. Food Res Int. 2020; 130:108933. PMID: 32156381.35. Rodríguez ML, Pérez S, Mena-Mollá S, Desco MC, Ortega ÁL. Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxid Med Cell Longev. 2019; 2019:4940825. PMID: 31814880.36. Gowd V, Kang Q, Wang Q, Wang Q, Chen F, Cheng KW. Resveratrol: evidence for its nephroprotective effect in diabetic nephropathy. Adv Nutr. 2020; 11:1555–1568. PMID: 32577714.37. Catrina SB, Zheng X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia. 2021; 64:709–716. PMID: 33496820.38. Lee EJ, Kang MK, Kim DY, Kim YH, Oh H, Kang YH. Chrysin inhibits advanced glycation end products-induced kidney fibrosis in renal mesangial cells and diabetic kidneys. Nutrients. 2018; 10:882. PMID: 29987200.39. Ravindran S, Munusamy S. Renoprotective mechanisms of sodium-glucose co-transporter 2 (SGLT2) inhibitors against the progression of diabetic kidney disease. J Cell Physiol. 2022; 237:1182–1205. PMID: 34713897.40. Alicic RZ, Cox EJ, Neumiller JJ, Tuttle KR. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021; 17:227–244. PMID: 33219281.41. Abbate M, Zoja C, Corna D, Rottoli D, Zanchi C, Azzollini N, Tomasoni S, Berlingeri S, Noris M, Morigi M, et al. Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J Am Soc Nephrol. 2008; 19:1158–1167. PMID: 18354030.42. Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond). 2014; 28:510–520. PMID: 24525867.43. Tan Y, Fukutomi A, Sun MT, Durkin S, Gilhotra J, Chan WO. Anti-VEGF crunch syndrome in proliferative diabetic retinopathy: a review. Surv Ophthalmol. 2021; 66:926–932. PMID: 33705807.44. Sulaiman RS, Basavarajappa HD, Corson TW. Natural product inhibitors of ocular angiogenesis. Exp Eye Res. 2014; 129:161–171. PMID: 25304218.45. Stompor-Gorący M, Bajek-Bil A, Machaczka M. Chrysin: perspectives on contemporary status and future possibilities as pro-health agent. Nutrients. 2021; 13:2038. PMID: 34198618.46. Zhu ZY, Wang WX, Wang ZQ, Chen LJ, Zhang JY, Liu XC, Wu SP, Zhang YM. Synthesis and antitumor activity evaluation of chrysin derivatives. Eur J Med Chem. 2014; 75:297–300. PMID: 24556144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of glucagon-like peptide 1 on salivary gland hypofunction in diabetic db/db mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation
- Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice
- An age-dependent alteration of the respiratory exchange ratio in the db/db mouse
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice