Diabetes Metab J.
2023 May;47(3):382-393. 10.4093/dmj.2022.0156.
Role of SUMO-Specific Protease 2 in Leptin-Induced Fatty Acid Metabolism in White Adipocytes
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea
- 3Department of Molecular Medicine and Biopharmaceutical Sciences, Seoul National University, Seoul, Korea
- KMID: 2542515
- DOI: http://doi.org/10.4093/dmj.2022.0156
Abstract
- Background
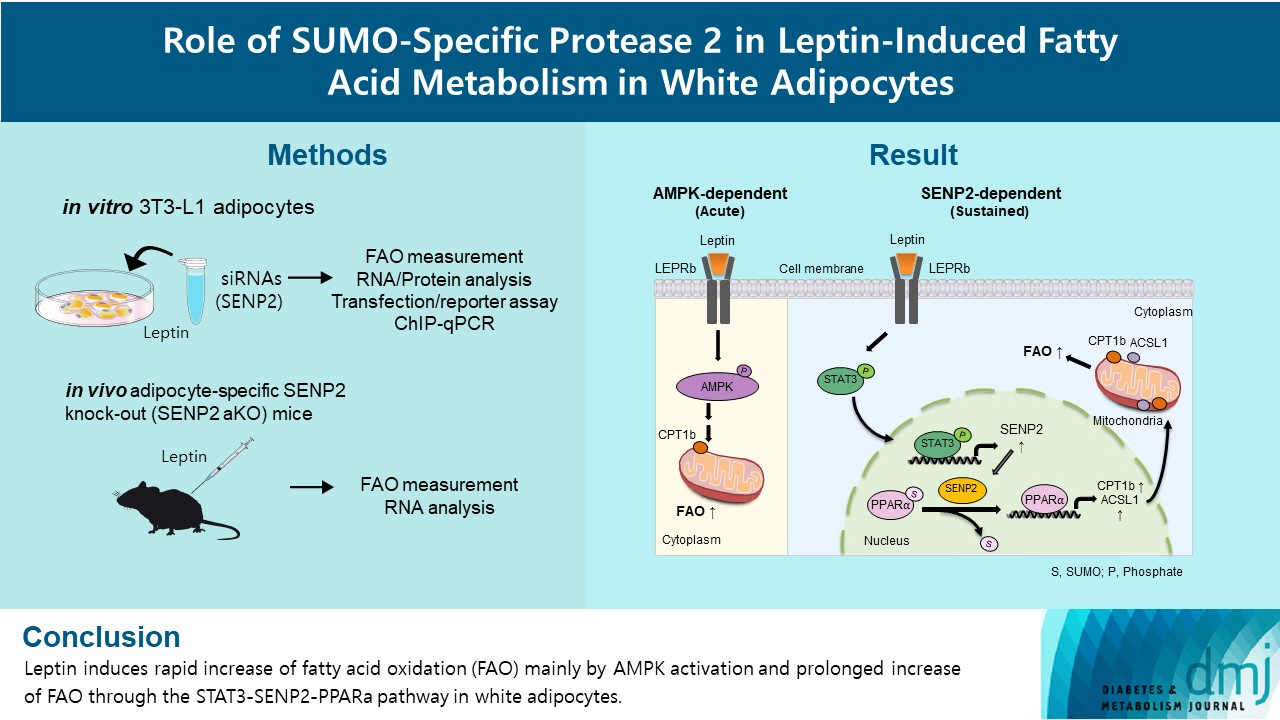
Leptin is a 16-kDa fat-derived hormone with a primary role in controlling adipose tissue levels. Leptin increases fatty acid oxidation (FAO) acutely through adenosine monophosphate-activated protein kinase (AMPK) and on delay through the SUMO-specific protease 2 (SENP2)–peroxisome proliferator-activated receptor δ/γ (PPARδ/γ) pathway in skeletal muscle. Leptin also directly increases FAO and decreases lipogenesis in adipocytes; however, the mechanism behind these effects remains unknown. Here, we investigated the role of SENP2 in the regulation of fatty acid metabolism by leptin in adipocytes and white adipose tissues.
Methods
The effects of leptin mediated by SENP2 on fatty acid metabolism were tested by siRNA-mediated knockdown in 3T3-L1 adipocytes. The role of SENP2 was confirmed in vivo using adipocyte-specific Senp2 knockout (Senp2-aKO) mice. We revealed the molecular mechanism involved in the leptin-induced transcriptional regulation of carnitine palmitoyl transferase 1b (Cpt1b) and long-chain acyl-coenzyme A synthetase 1 (Acsl1) using transfection/reporter assays and chromatin immunoprecipitation.
Results
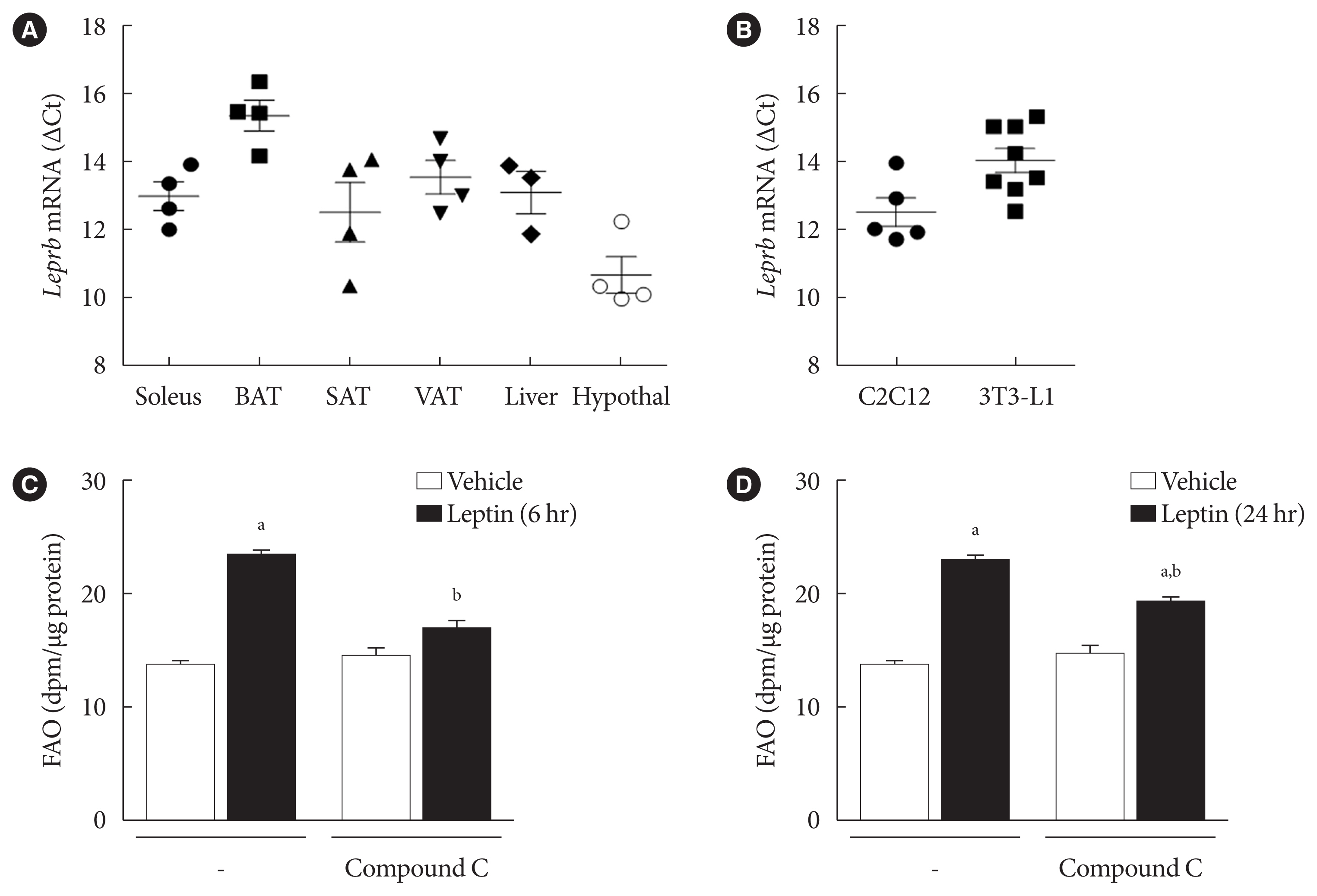
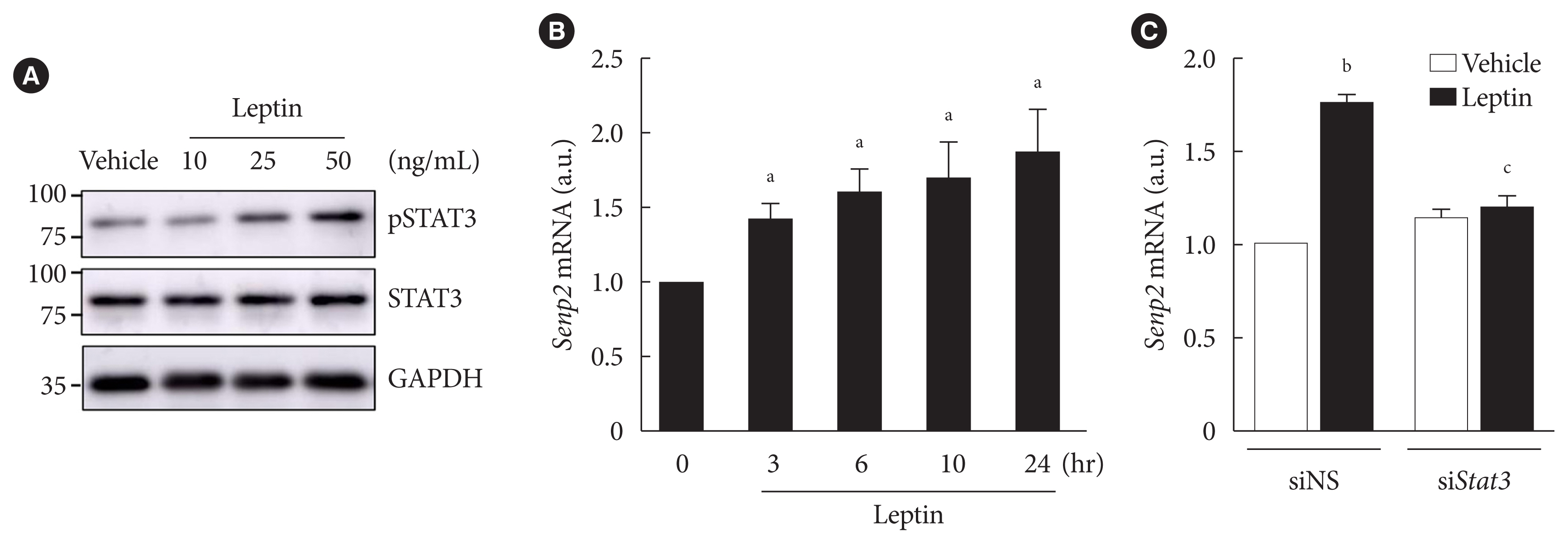
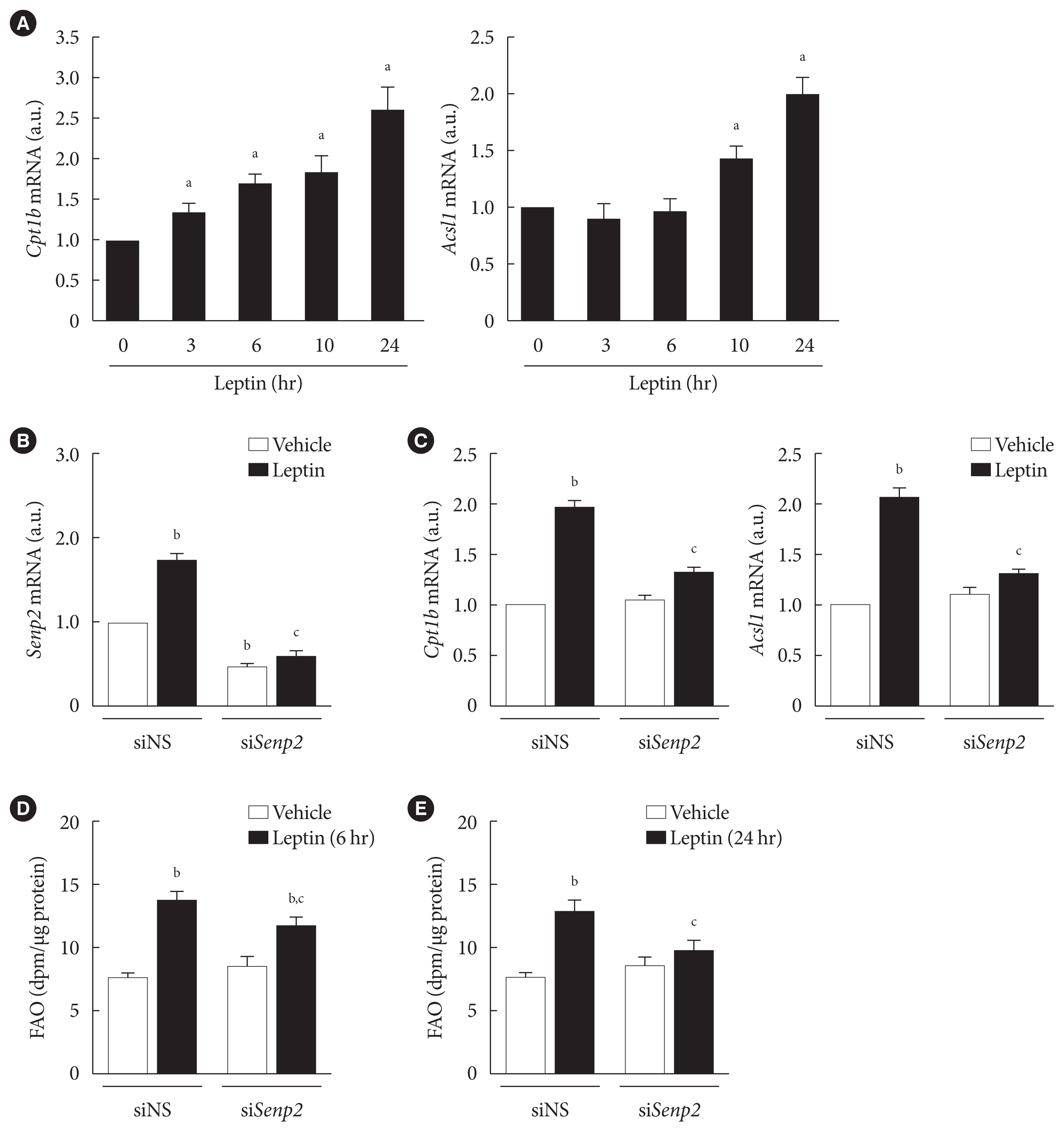
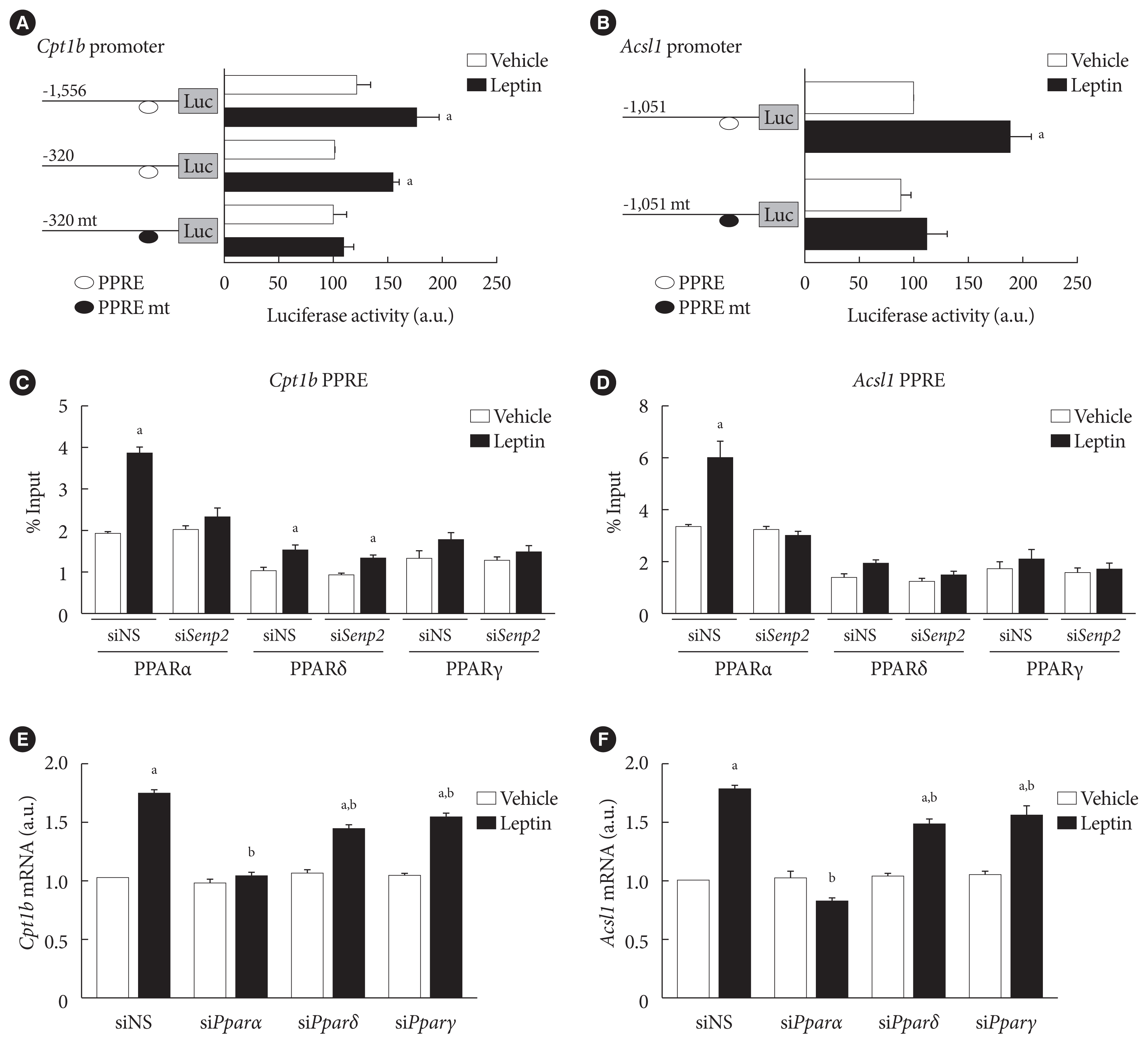
SENP2 mediated the increased expression of FAO-associated enzymes, CPT1b and ACSL1, which peaked 24 hours after leptin treatment in adipocytes. In contrast, leptin stimulated FAO through AMPK during the initial several hours after treatment. In white adipose tissues, FAO and mRNA levels of Cpt1b and Acsl1 were increased by 2-fold 24 hours after leptin injection in control mice but not in Senp2-aKO mice. Leptin increased PPARα binding to the Cpt1b and Acsl1 promoters in adipocytes through SENP2.
Conclusion
These results suggest that the SENP2-PPARα pathway plays an important role in leptin-induced FAO in white adipocytes.
Keyword
Figure
Reference
-
1. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 395:763–70.2. Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002; 415:339–43.3. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995; 83:1263–71.4. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996; 379:632–5.5. Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996; 14:95–7.
Article6. Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 1996; 93:6231–5.7. Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996; 387:113–6.
Article8. Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci U S A. 1997; 94:7001–5.9. Siegrist-Kaiser CA, Pauli V, Juge-Aubry CE, Boss O, Pernin A, Chin WW, et al. Direct effects of leptin on brown and white adipose tissue. J Clin Invest. 1997; 100:2858–64.
Article10. Huynh FK, Neumann UH, Wang Y, Rodrigues B, Kieffer TJ, Covey SD. A role for hepatic leptin signaling in lipid metabolism via altered very low density lipoprotein composition and liver lipase activity in mice. Hepatology. 2013; 57:543–54.
Article11. Moran O, Phillip M. Leptin: obesity, diabetes and other peripheral effects: a review. Pediatr Diabetes. 2003; 4:101–9.12. Jiang L, Wang Q, Yu Y, Zhao F, Huang P, Zeng R, et al. Leptin contributes to the adaptive responses of mice to high-fat diet intake through suppressing the lipogenic pathway. PLoS One. 2009; 4:e6884.
Article13. Chang HM, Yeh ETH. SUMO: from bench to bedside. Physiol Rev. 2020; 100:1599–619.
Article14. Mendes AV, Grou CP, Azevedo JE, Pinto MP. Evaluation of the activity and substrate specificity of the human SENP family of SUMO proteases. Biochim Biophys Acta. 2016; 1863:139–47.
Article15. Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol. 2012; 13:755–66.
Article16. Koo YD, Choi JW, Kim M, Chae S, Ahn BY, Kim M, et al. SUMO-specific protease 2 (SENP2) is an important regulator of fatty acid metabolism in skeletal muscle. Diabetes. 2015; 64:2420–31.
Article17. Muoio DM, Dohm GL, Fiedorek FT Jr, Tapscott EB, Coleman RA. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997; 46:1360–3.18. Koo YD, Lee JS, Lee SA, Quaresma PG, Bhat R, Haynes WG, et al. SUMO-specific protease 2 mediates leptin-induced fatty acid oxidation in skeletal muscle. Metabolism. 2019; 95:27–35.
Article19. Pico C, Palou M, Pomar CA, Rodriguez AM, Palou A. Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord. 2022; 23:13–30.
Article20. Stern JH, Rutkowski JM, Scherer PE. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016; 23:770–84.
Article21. Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015; 163:84–94.
Article22. Pereira S, O’Dwyer SM, Webber TD, Baker RK, So V, Ellis CE, et al. Metabolic effects of leptin receptor knockdown or reconstitution in adipose tissues. Sci Rep. 2019; 9:3307.23. William WN Jr, Ceddia RB, Curi R. Leptin controls the fate of fatty acids in isolated rat white adipocytes. J Endocrinol. 2002; 175:735–44.
Article24. Wang MY, Lee Y, Unger RH. Novel form of lipolysis induced by leptin. J Biol Chem. 1999; 274:17541–4.
Article25. Lee JS, Chae S, Nan J, Koo YD, Lee SA, Park YJ, et al. SENP2 suppresses browning of white adipose tissues by de-conjugating SUMO from C/EBPβ. Cell Rep. 2022; 38:110408.
Article26. Ceddia RB, William WN Jr, Lima FB, Flandin P, Curi R, Giacobino JP. Leptin stimulates uncoupling protein-2 mRNA expression and Krebs cycle activity and inhibits lipid synthesis in isolated rat white adipocytes. Eur J Biochem. 2000; 267:5952–8.
Article27. Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C, et al. JAK/STAT3-regulated fatty acid β-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 2018; 27:136–50.28. Brun RP, Tontonoz P, Forman BM, Ellis R, Chen J, Evans RM, et al. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996; 10:974–84.
Article29. Jeong HW, Lee JW, Kim WS, Choe SS, Kim KH, Park HS, et al. A newly identified CG301269 improves lipid and glucose metabolism without body weight gain through activation of peroxisome proliferator-activated receptor alpha and gamma. Diabetes. 2011; 60:496–506.
Article30. Pourcet B, Pineda-Torra I, Derudas B, Staels B, Glineur C. SUMOylation of human peroxisome proliferator-activated receptor alpha inhibits its trans-activity through the recruitment of the nuclear corepressor NCoR. J Biol Chem. 2010; 285:5983–92.31. Goto T, Lee JY, Teraminami A, Kim YI, Hirai S, Uemura T, et al. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J Lipid Res. 2011; 52:873–84.
Article32. Lee JY, Hashizaki H, Goto T, Sakamoto T, Takahashi N, Kawada T. Activation of peroxisome proliferator-activated receptor-α enhances fatty acid oxidation in human adipocytes. Biochem Biophys Res Commun. 2011; 407:818–22.
Article33. Hinds TD Jr, Kipp ZA, Xu M, Yiannikouris FB, Morris AJ, Stec DF, et al. Adipose-specific PPARα knockout mice have increased lipogenesis by PASK-SREBP1 signaling and a polarity shift to inflammatory macrophages in white adipose tissue. Cells. 2021; 11:4.34. Commins SP, Watson PM, Frampton IC, Gettys TW. Leptin selectively reduces white adipose tissue in mice via a UCP1-dependent mechanism in brown adipose tissue. Am J Physiol Endocrinol Metab. 2001; 280:E372–7.
Article35. Li YC, Zheng XL, Liu BT, Yang GS. Regulation of ATGL expression mediated by leptin in vitro in porcine adipocyte lipolysis. Mol Cell Biochem. 2010; 333:121–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Butyrate regulates leptin expression through different signaling pathways in adipocytes
- Role of TRPV4 Channel in Human White Adipocytes Metabolic Activity
- Eosinophils and Type 2 Cytokine Signaling in Macrophages Support the Biogenesis of Cold-induced Beige Fat
- Bone and Energy Metabolism
- Regulation of leptin gene expression by insulin and growth hormone in mouse adipocytes