J Vet Sci.
2011 Dec;12(4):319-323. 10.4142/jvs.2011.12.4.319.
Butyrate regulates leptin expression through different signaling pathways in adipocytes
- Affiliations
-
- 1Department of Biochemistry, Faculty of Veterinary Medicine, Benha Universities, P.O 13736, Egypt. mohamedsoliman8896@yahoo.com
- 2Department of Biotechnology, Faculty of Applied Medical sciences, Taif University, P.O. 21974, Saudi Arabia.
- 3Department of Biochemistry, Faculty of Veterinary Medicine, Minufiya Universities, P.O 13736, Egypt.
- 4Department of Biotechnology, Turabah and Faculty of Science, Taif University, P.O. 21974, Saudi Arabia.
- 5Zoology Department, Faculty of Science at Aswan, P.O. 81528, South Valley University, Egypt.
- 6Medical Laboratories Department, College of Science in AlZulfi, Majmaah University, P.O. 1712-11932, Saudi Arabia.
- 7Department of Physiology, Faculty of Veterinary Medicine, Benha Universities, P.O 13736, Egypt.
- KMID: 1365014
- DOI: http://doi.org/10.4142/jvs.2011.12.4.319
Abstract
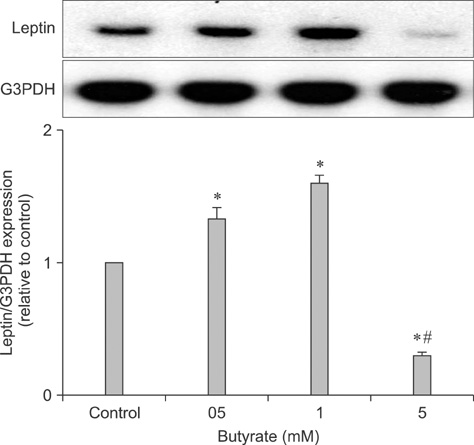
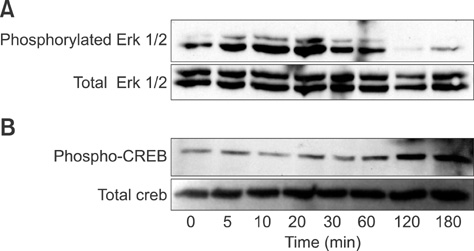
- Leptin is an adipocytokine that regulates body weight, and maintains energy homeostasis by promoting reduced food intake and increasing energy expenditure. Leptin expression and secretion is regulated by various factors including hormones and fatty acids. Butyrate is a short-chain fatty acid that acts as source of energy in humans. We determined whether this fatty acid can play a role in leptin expression in fully differentiated human adipocytes. Mature differentiated adipocytes were incubated with or without increasing concentrations of butyrate. RNA was extracted and leptin mRNA expression was examined by Northern blot analysis. Moreover, the cells were incubated with regulators that may affect signals which may alter leptin expression and analyzed with Northern blotting. Butyrate stimulated leptin expression, and stimulated mitogen activated protein kinase (MAPK) and phospho-CREB signaling in a time-dependent manner. Prior treatment of the cells with signal transduction inhibitors as pertusis toxin, Gi protein antagonist, PD98059 (a MAPK inhibitor), and wortmannin (a PI3K inhibitor) abolished leptin mRNA expression. These results suggest that butyrate can regulate leptin expression in humans at the transcriptional level. This is accomplished by: 1) Gi protein-coupled receptors specific for short-chain fatty acids, and 2) MAPK and phosphatidylinositol-3-kinase (PI3K) signaling pathways.
Keyword
MeSH Terms
-
Adipocytes/*metabolism
Azo Compounds
Butyric Acid/*pharmacology
CREB-Binding Protein/genetics/metabolism
Cell Differentiation
Cells, Cultured
Gene Expression Regulation/*drug effects/physiology
Humans
Leptin/genetics/*metabolism
Mitogen-Activated Protein Kinase Kinases/genetics/metabolism
Phosphatidylinositol 3-Kinases/genetics/metabolism
RNA, Messenger/genetics/metabolism
Signal Transduction/*physiology
Staining and Labeling
Figure
Reference
-
1. Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000. 62:413–437.
Article2. Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996. 6:1170–1180.
Article3. Blaukat A, Barac A, Cross MJ, Offermanns S, Dikic I. G protein-coupled receptor-mediated mitogen-activated protein kinase activation through cooperation of Gαq and Gαi signals. Mol Cell Biol. 2000. 20:6837–6848.
Article4. Coradini D, Pellizzaro C, Marimpietri D, Abolafio G, Daidone MG. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif. 2000. 33:139–146.
Article5. Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000. 68:437–446.6. Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005. 146:5092–5099.
Article7. Katoh K, Tsuda T. Effects of acetylcholine and short-chain fatty acids on acinar cells of the exocrine pancreas in sheep. J Physiol. 1984. 356:479–489.
Article8. Lazar MA. Sodium butyrate selectively alters thyroid hormone receptor gene expression in GH3 cells. J Biol Chem. 1990. 265:17474–17477.
Article9. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996. 379:632–635.
Article10. Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997. 3:1029–1033.
Article11. Matsunaga N, Nam KT, Kuhara T, Oda S, Ohneda A, Sasaki Y. Inhibition of GH releasing factor (GRF)-induced GH secretion by intraruminal infusion of volatile fatty acids (VFA) in sheep. Endocr J. 1997. 44:133–140.
Article12. Matsunaga N, Nam KT, Kuhara T, Oda S, Ohneda A, Sasaki Y. Inhibitory effect of volatile fatty acids on GRF-induced GH secretion in sheep. Endocr J. 1993. 40:529–537.
Article13. Mitsuhashi T, Uchimura H, Takaku F. n-Butyrate increases the level of thyroid hormone nuclear receptor in non-pituitary cultured cells. J Biol Chem. 1987. 262:3993–3999.
Article14. Pudel V, Ellrott T. Social and political aspects of adiposis. Chirurg. 2005. 76:639–646.15. Reincke M, Beuschlein F, Slawik M. Obesity 2006. MMW Fortschr Med. 2006. 148:20–24.16. Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997. 82:579–584.17. Schwartz MW, Baskin DG, Kaiyala KJ, Woods SC. Model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr. 1999. 69:584–596.
Article18. Soliman M, Kimura K, Ahmed M, Yamaji D, Matsushita Y, Okamatsu-Ogura Y, Makondo K, Saito M. Inverse regulation of leptin mRNA expression by short- and long-chain fatty acids in cultured bovine adipocytes. Domest Anim Endocrinol. 2007. 33:400–409.
Article19. Tabuchi Y, Arai Y, Kondo T, Takeguchi N, Asano S. Identification of genes responsive to sodium butyrate in colonic epithelial cells. Biochem Biophys Res Commun. 2002. 293:1287–1294.
Article20. Tartaglia LA. The leptin receptor. J Biol Chem. 1997. 272:6093–6096.
Article21. Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995. 83:1263–1271.
Article22. Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001. 81:1031–1064.
Article23. Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004. 101:1045–1050.
Article24. Yonekura S, Senoo T, Kobayashi Y, Yonezawa T, Katoh K, Obara Y. Effects of acetate and butyrate on the expression of leptin and short-form leptin receptor in bovine and rat anterior pituitary cells. Gen Comp Endocrinol. 2003. 133:165–172.
Article25. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994. 372:425–432.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Leptin-signal transduction pathways and relationship with cancer development
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling
- Regulation of leptin gene expression by insulin and growth hormone in mouse adipocytes
- Signaling Role of Adipocyte Leptin in Prostate Cell Proliferation Induced by Trichomonas vaginalis
- The Effects of Ginseng Saponin-Re, Rc and Green Tea Catechine; ECGC (Epigallocatechin Gallate) on Leptin, Hormone Sensitive Lipase and Resistin mRNA Expressions in 3T3-L1 Adipocytes