Cancer Res Treat.
2023 Apr;55(2):542-550. 10.4143/crt.2022.834.
Androgen Receptor as a Predictive Marker for Pathologic Complete Response in Hormone Receptor–Positive and HER-2–Negative Breast Cancer with Neoadjuvant Chemotherapy
- Affiliations
-
- 1Center for Breast Cancer, National Cancer Center, Goyang, Korea
- 2Biostatistics Collaboration Team, Research Core Center, Research Institute of National Cancer Center, Goyang, Korea
- 3Department of Pathology, National Cancer Center, Goyang, Korea
- 4Cancer Healthcare Research Branch, Research Institute of National Cancer Center, Goyang, Korea
- KMID: 2541241
- DOI: http://doi.org/10.4143/crt.2022.834
Abstract
- Purpose
This study investigated pathological complete response (pCR) according to androgen receptor (AR) in breast cancer patients undergoing neoadjuvant chemotherapy and estimated the relationship between AR expression and clinicopathological factors.
Materials and Methods
We identified 624 breast cancer patients who underwent surgery after neoadjuvant chemotherapy at the National Cancer Center in Goyang, Korea from April 2016 to October 2019. We retrospectively collected the clinicopathologic information and AR expression results and analyzed the data according to cancer stage, hormonal receptor (HR) status, human epidermal growth factor receptor 2 (HER2) status, tumor subtype, and pCR.
Results
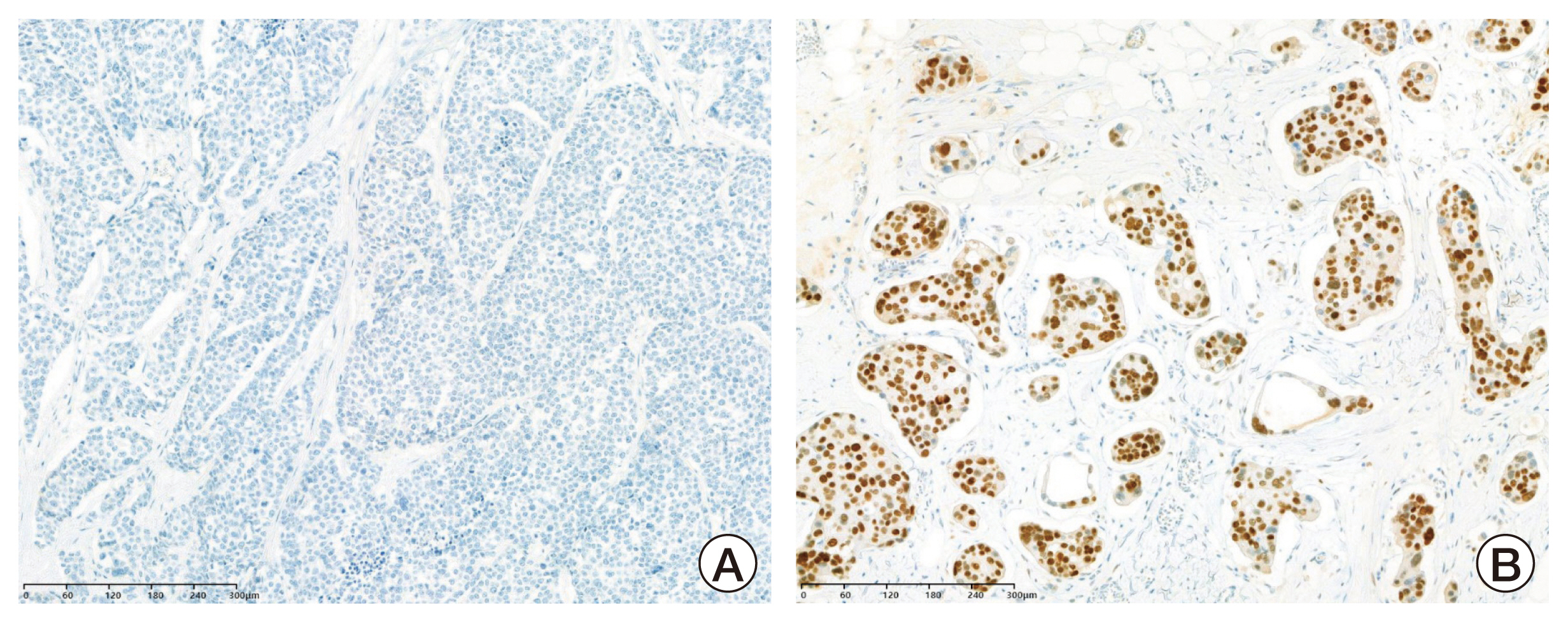
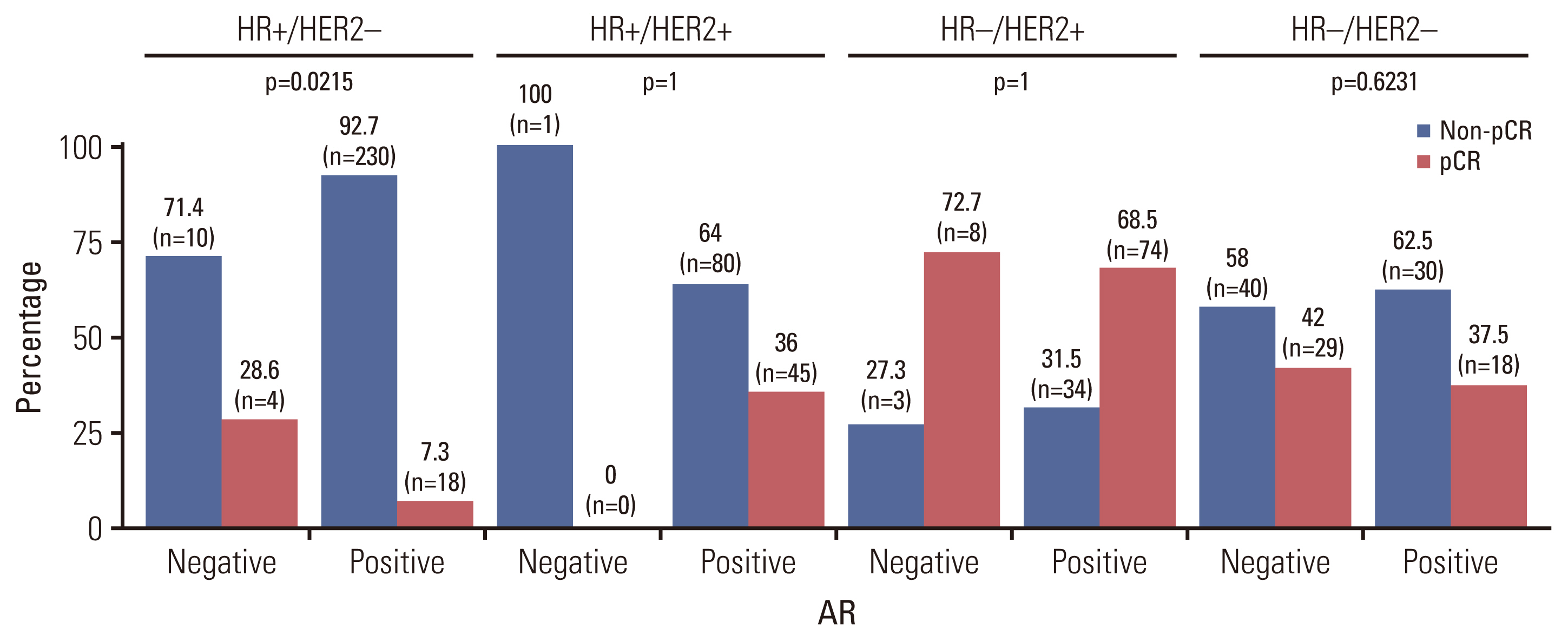
Among the 624 breast cancer patients, 529 (84.8%) were AR-positive (AR+) patients and 95 (15.2%) were AR-negative (AR–) patients. AR+ patients showed more estrogen receptor (ER) positivity, progesterone receptor (PR) positivity, HER2-positivity, and HR-positive and HER2-negative (HR+/HER2–) subtype. The rate of pCR was 31.4% (196/624). AR– patients had a significantly higher rate of pCR than AR+ patients (AR– 43.2% vs. AR+ 29.3%, p=0.007). The tumor factors associated with pCR were early stage, histologic grade 3, ER-negative, PR-negative, AR-negative, HER2-positive, and high Ki-67 values. In univariable analysis, AR+ significantly decreased the state of pCR (odds ratio, 0.546; 95% confidence interval, 0.349 to 0.853; p=0.008). According to tumor subtype, AR– tumor showed higher pCR rate in HR+/HER2– subtype (AR– 28.6% vs. AR+ 7.3%, p=0.022).
Conclusion
AR expression is predominant in the HR+/HER2– subtype. AR– is significantly associated with the pCR rate in breast cancer patients, especially within HR+/HER2– subtype. When determining neoadjuvant chemotherapy for the HR+/HER2– subtype, AR expression can be considered as a pCR predictive marker.
Figure
Reference
-
References
1. National Comprehensive Cancer Network. Breast cancer (version 5, 2021) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2021. [cited 2021 Jun 28]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .2. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–72.
Article3. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–804.
Article4. Gonzalez LO, Corte MD, Vazquez J, Junquera S, Sanchez R, Alvarez AC, et al. Androgen receptor expresion in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer. 2008; 8:149.
Article5. Moinfar F, Okcu M, Tsybrovskyy O, Regitnig P, Lax SF, Weybora W, et al. Androgen receptors frequently are expressed in breast carcinomas: potential relevance to new therapeutic strategies. Cancer. 2003; 98:703–11.
Article6. Gerratana L, Basile D, Buono G, De Placido S, Giuliano M, Minichillo S, et al. Androgen receptor in triple negative breast cancer: a potential target for the targetless subtype. Cancer Treat Rev. 2018; 68:102–10.
Article7. Kuenen-Boumeester V, Van der Kwast TH, Claassen CC, Look MP, Liem GS, Klijn JG, et al. The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer. 1996; 32A:1560–5.
Article8. Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003; 120:725–31.
Article9. Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009; 15:2472–8.
Article10. Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106:djt319.
Article11. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–68.12. Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014; 138:241–56.
Article13. Yang Y, Min A, Lee KH, Ryu HS, Kim TY, Woo GU, et al. Prognostic role of androgen receptor expression in surgically resected early breast cancer patients. J Breast Cancer. 2020; 23:182–93.
Article14. Dieci MV, Tsvetkova V, Griguolo G, Miglietta F, Mantiero M, Tasca G, et al. Androgen receptor expression and association with distant disease-free survival in triple negative breast cancer: analysis of 263 Patients Treated With Standard Therapy for Stage I–III Disease. Front Oncol. 2019; 9:452.15. Akashi M, Yamaguchi R, Kusano H, Ogasawara S, Abe E, Obara H, et al. Androgen receptor expression is useful to predict the therapeutic effect in HER2-positive breast carcinoma. Breast Cancer Res Treat. 2020; 184:277–85.
Article16. Kensler KH, Regan MM, Heng YJ, Baker GM, Pyle ME, Schnitt SJ, et al. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: results from the Breast International Group Trial 1–98. Breast Cancer Res. 2019; 21:30.
Article17. Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011; 17:1867–74.
Article18. Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011; 22:1755–62.
Article19. Witzel I, Loibl S, Wirtz R, Fasching PA, Denkert C, Weber K, et al. Androgen receptor expression and response to chemotherapy in breast cancer patients treated in the neoadjuvant TECHNO and PREPARE trial. Br J Cancer. 2019; 121:1009–15.
Article20. Nakashoji A, Matsui A, Nagayama A, Iwata Y, Sasahara M, Murata Y. Clinical predictors of pathological complete response to neoadjuvant chemotherapy in triple-negative breast cancer. Oncol Lett. 2017; 14:4135–41.
Article21. Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–81.
Article22. Loibl S, Muller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011; 130:477–87.
Article23. Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013; 19:5533–40.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ki-67 as a Predictor of Response to Neoadjuvant Chemotherapy in Breast Cancer Patients
- Human Epidermal Growth Factor Receptor 2-positive Mucinous Carcinoma with Signet Ring Cell Differentiation, Which Showed Complete Response after Neoadjuvant Chemotherapy
- Durable Response of Androgen Receptor-Positive Male Breast Cancer to Goserelin
- Alteration of Estrogen Receptor, Progesterone Receptor, and HER-2 Expression in Breast Cancer after Neoadjuvant Chemotherapy
- Pathologic Findings of Residual Tumor according to the Response Rate after Neoadjuvant Chemotherapy for Breast Cancer