Obstet Gynecol Sci.
2023 Mar;66(2):100-106. 10.5468/ogs.22267.
Growth inhibition by fusidic acid in cervical, thyroid, and breast carcinoma cell lines
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Hanyang University College of Medicine, Seoul, Korea
- 2Department Departments of Surgery, Konkuk University School of Medicine, Seoul, Korea
- 3Puricellmania Corporation, Seoul, Korea
- KMID: 2540479
- DOI: http://doi.org/10.5468/ogs.22267
Abstract
Objective
We investigated the effects of fusidic acid (FA) on human cervical, thyroid, and breast carcinoma cell lines to determine the potential usefulness of FA in cancer treatment.
Methods
Six cancer cell lines (cervical cancer: Caski, HeLa; thyroid cancer: 8505C, TPC1; and breast cancer: MCF-7, MDA-MB-231) were treated with FA. Furthermore the changes in cell growth, cell cycle duration, and extent of apoptosis were analyzed.
Results
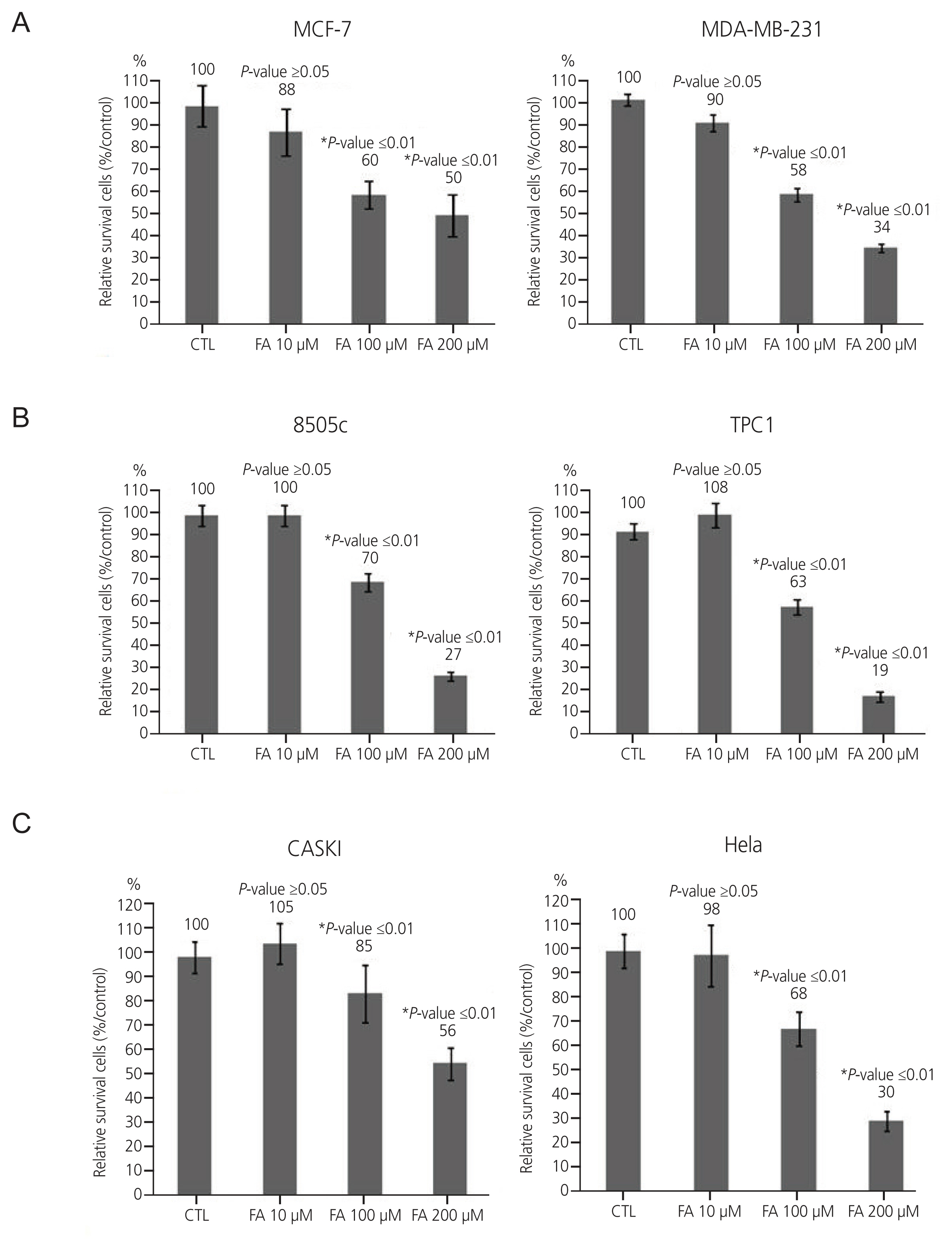
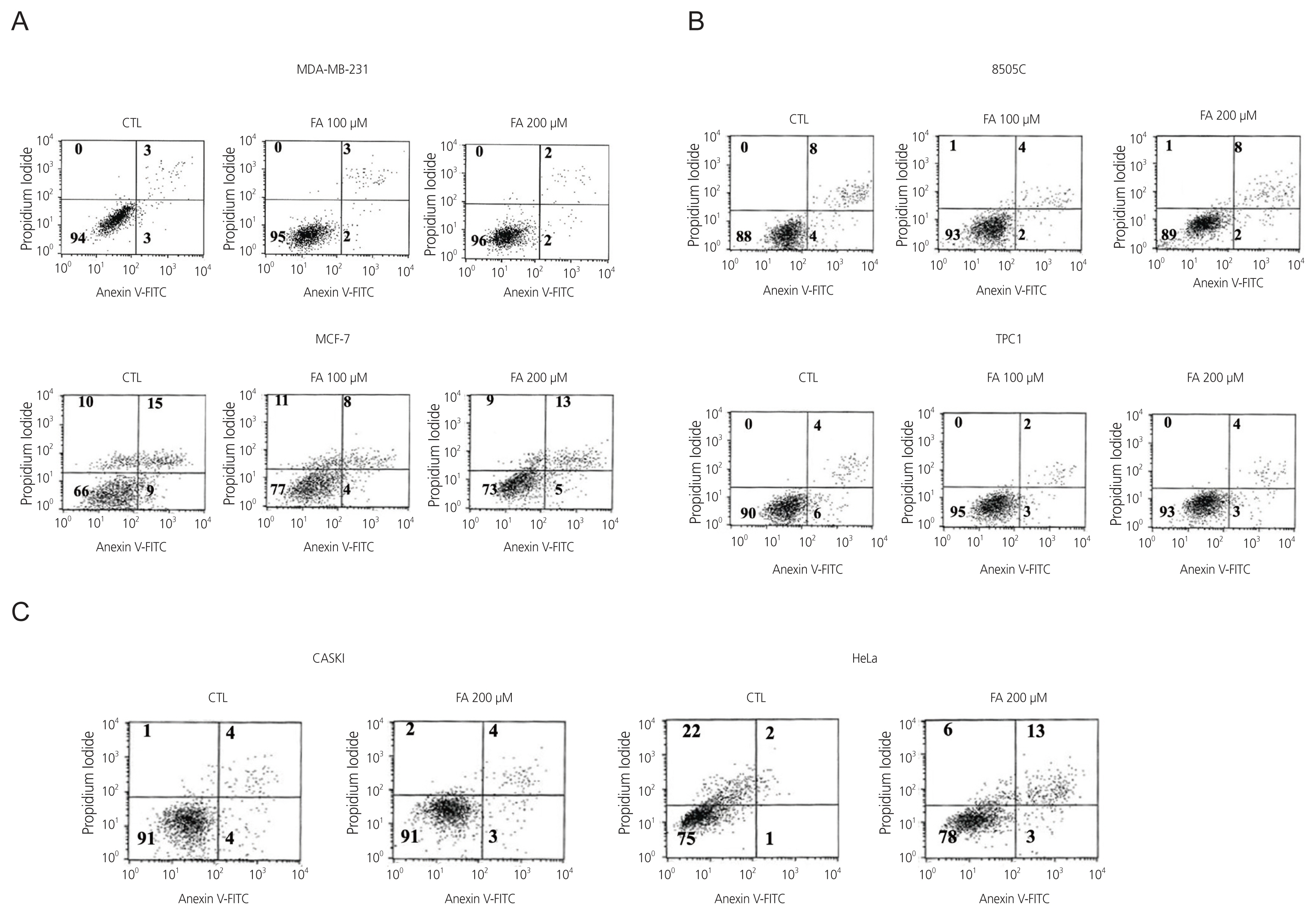
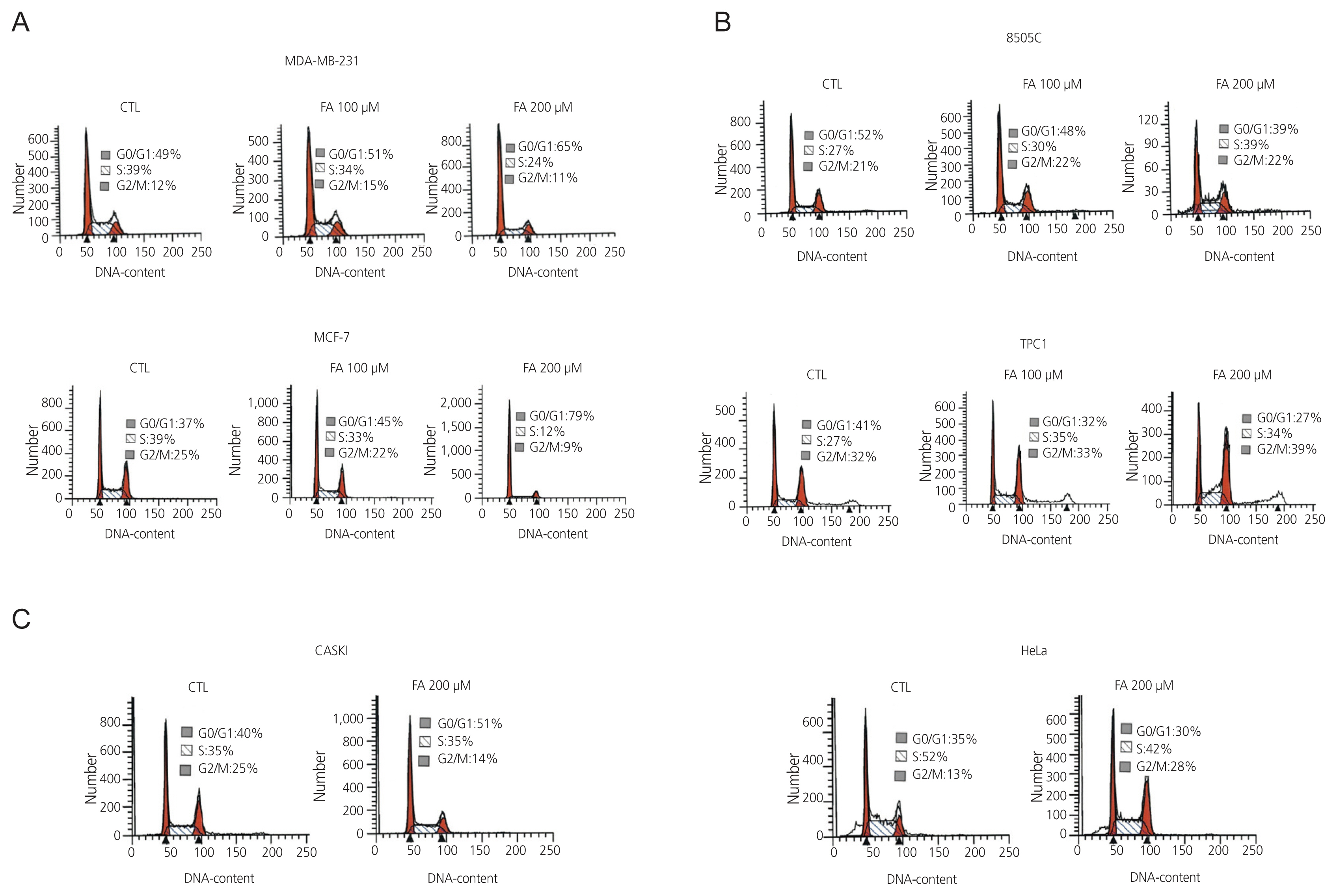
After FA treatment, the cancer cells showed a decrease in growth rate. In the cell death assay, the cell populations were similar in each cell type after treatment with FA, indicating that growth inhibition by FA was not related to the induction of apoptosis. FA induced cell cycle arrest at a dose that inhibited growth rate, which varied in different cell types. G0/G1 phase arrest occurs in breast cancer, S phase arrest in 8505C thyroid cancer, and G2/M phase arrest in cervical cancer. These results indicate that FA reduces growth rates by inducing cell cycle arrest.
Conclusion
FA treatment can interfere with cell proliferation by inducing cell cycle arrest in human cervical, thyroid, and breast carcinoma cell lines. Thus, FA can be useful in treating human cervical, thyroid, and breast carcinomas.
Keyword
Figure
Reference
-
References
1. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.2. Korea Central Cancer Registry. Annual report of cancer statistics in Korea in 2019 [Internet]. Goyang: National Cancer Information Center;c2019. [cited 2022 Oct 27]. Available from: https://ncc.re.kr/cancerStatsView.ncc?bbsnum=578&searchKey=total&searchValue=&pageNum=1 .3. Fernandes P. Fusidic acid: a bacterial elongation factor inhibitor for the oral treatment of acute and chronic staphylococcal infections. Cold Spring Harb Perspect Med. 2016; 6:a025437.4. Savelsbergh A, Rodnina MV, Wintermeyer W. Distinct functions of elongation factor G in ribosome recycling and translocation. RNA. 2009; 15:772–80.5. Godtfredsen WO, Jahnsen S, Lorck H, Roholt K, Tybring L. Fusidic acid: a new antibiotic. Nature. 1962; 193:987.6. Harvey CL, Knight SG, Sih CJ. On the mode of action of fusidic acid. Biochemistry. 1966; 5:3320–7.7. Howden BP, Grayson ML. Dumb and dumber--the potential waste of a useful antistaphylococcal agent: emerging fusidic acid resistance in Staphylococcus aureus. Clin Infect Dis. 2006; 42:394–400.8. Arigoni D, Vondaehne W, Godtfredsen WO, Marquet A, Melera A. The location of the ring C hydroxyl group in fusidic acid. Experientia. 1963; 19:521–2.9. Yamaki H. Inhibition of protein synthesis by fusidic and helvolinic acids, steroidal antibiotics. J Antibiot (Tokyo). 1965; 18:228–32.10. Koripella RK, Chen Y, Peisker K, Koh CS, Selmer M, Sanyal S. Mechanism of elongation factor-G-mediated fusidic acid resistance and fitness compensation in Staphylococcus aureus. J Biol Chem. 2012; 287:30257–67.11. White SJ, Kasman LM, Kelly MM, Lu P, Spruill L, McDermott PJ, et al. Doxorubicin generates a proapoptotic phenotype by phosphorylation of elongation factor 2. Free Radic Biol Med. 2007; 43:1313–21.12. Huizing MT, Misser VH, Pieters RC, ten Bokkel Huinink WW, Veenhof CH, Vermorken JB, et al. Taxanes: a new class of antitumor agents. Cancer Invest. 1995; 13:381–404.13. White-Gilbertson S, Kurtz DT, Voelkel-Johnson C. The role of protein synthesis in cell cycling and cancer. Mol Oncol. 2009; 3:402–8.14. Thornes RD, Sheehan MV. “Fucidin” therapy in carcinoma of the breast. (Preliminary report). J Ir Med Assoc. 1966; 58:52–4.15. New Zealand Medicines and Medical Devices Safety Authority. Fucidin patient information leaflet [Internet]. Wellington (NZ): New Zealand Medicines and Medical Devices Safety Authority;c2017. [cited 2022 Oct 27]. Available from: https://www.medsafe.govt.nz/consumers/cmi/f/Fucidin.pdf .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Cell Growth and Suppression of c-myc Gene Expression by Transforming Growth Factor-beta1 in Cervical Carcinoma Cell Lines
- The Mechanisms of Growth Inhibition by Transforming Growth Factor-beta1 in Cervical Carcinoma Cell Lines
- A Case of Squamous Cell Carcinoma of Cervical Esophagus with Metastasis to Thyroid Gland
- Apoptosis and Cell Cycle Arrest with EGF, TGF- a and TGF- 8 in Cervical Cancer Cell Lines
- Correlation between Retinoic Acid Sensitivity and IGFBP-3, AFP Protein Expression in Hepatoma Cell Lines