Acute Crit Care.
2022 Nov;37(4):502-515. 10.4266/acc.2022.00780.
Lung ultrasound for evaluation of dyspnea: a pictorial review
- Affiliations
-
- 1Department of Radiodiagnosis, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India
- 2Department of Pediatrics, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India
- 3Department of Pulmonary Medicine, Maulana Azad Medical College and Lok Nayak Hospital, New Delhi, India
- KMID: 2540138
- DOI: http://doi.org/10.4266/acc.2022.00780
Abstract
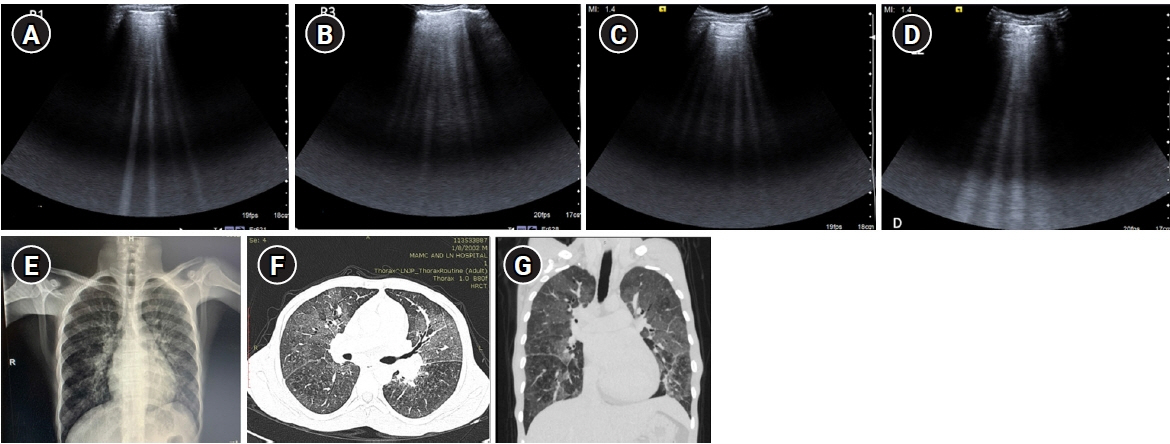
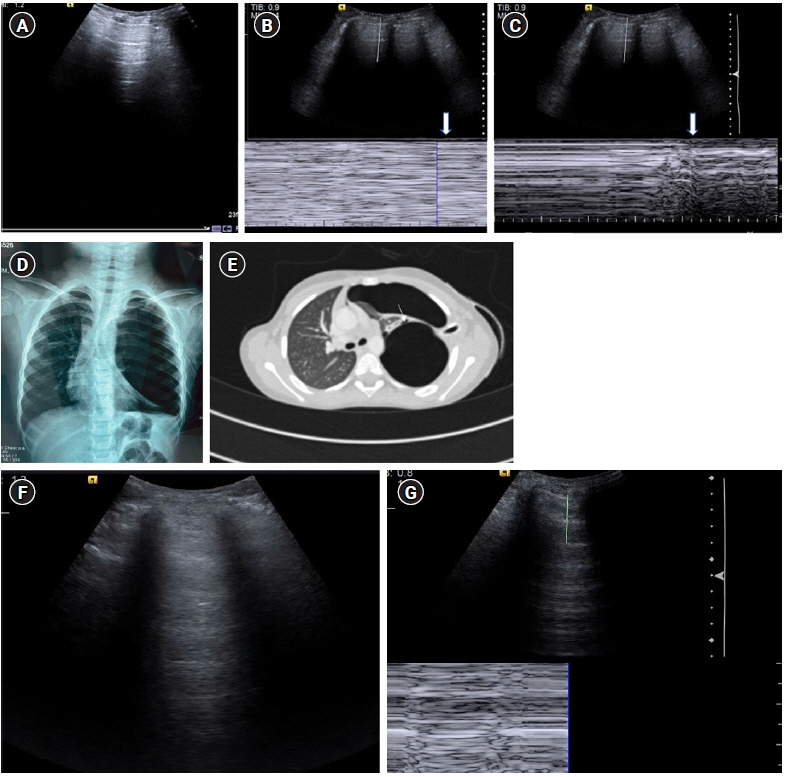
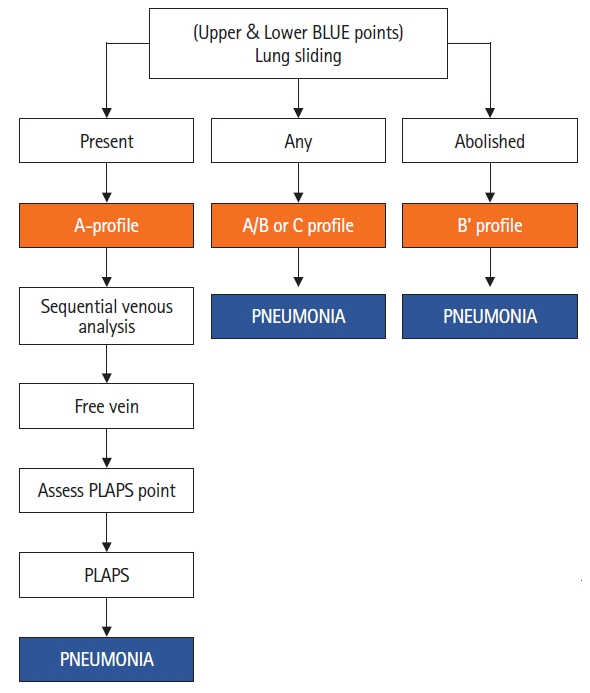
- Lung ultrasound is based on the analysis of ultrasound artifacts generated by the pleura and air within the lungs. In recent years, lung ultrasound has emerged as an important alternative for quick evaluation of the patient at the bedside. Several techniques and protocols for performing lung ultrasound have been described in the literature, with the most popular one being the Bedside Lung Ultrasound in Emergency (BLUE) protocol which can be utilized to diagnose the cause of acute dyspnea at the bedside. We attempt to provide a simplified approach to understanding the physics behind the artifacts used in lung ultrasound, the imaging techniques, and the application of the BLUE protocol to diagnose the commonly presenting causes of acute dyspnea.
Keyword
Figure
Reference
-
1. Lichtenstein DA. BLUE protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015; 147:1659–70.2. Feldman MK, Katyal S, Blackwood MS. US artifacts. Radiographics. 2009; 29:1179–89.
Article3. Baad M, Lu ZF, Reiser I, Paushter D. Clinical significance of US artifacts. Radiographics. 2017; 37:1408–23.
Article4. Gargani L, Volpicelli G. How I do it: lung ultrasound. Cardiovasc Ultrasound. 2014; 12:25.
Article5. Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol. 2004; 93:1265–70.
Article6. Laursen CB, Rahman NM, Volpicelli G. Thoracic ultrasound. Lausanne: European Respiratory Society;2018.7. Lichtenstein D. Lung ultrasound in the critically ill: The BLUE protocol. Switzerland: Springer;2016.8. Lichtenstein DA, Menu Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill: lung sliding. Chest. 1995; 108:1345–8.
Article9. Zanobetti M, Scorpiniti M, Gigli C, Nazerian P, Vanni S, Innocenti F, et al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest. 2017; 151:1295–301.
Article10. Volpicelli G, Mussa A, Garofalo G, Cardinale L, Casoli G, Perotto F, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006; 24:689–96.
Article11. Reissig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease: the role of comet tail artifacts. J Ultrasound Med. 2003; 22:173–80.12. Gargani L, Doveri M, D'Errico L, Frassi F, Bazzichi ML, Delle Sedie A, et al. Ultrasound lung comets in systemic sclerosis: a chest sonography hallmark of pulmonary interstitial fibrosis. Rheumatology (Oxford). 2009; 48:1382–7.
Article13. Copetti R, Copetti P, Soldati G. Ultrasound pattern in pulmonary fibrosis: have the vertical artifacts disappeared? Ultrasound Med Biol. 2010; 36:356–7.
Article14. Reissig A, Copetti R. Lung ultrasound in community-acquired pneumonia and in interstitial lung diseases. Respiration. 2014; 87:179–89.
Article15. Buda N, Piskunowicz M, Porzezińska M, Kosiak W, Zdrojewski Z. Lung ultrasonography in the evaluation of interstitial lung disease in systemic connective tissue diseases: criteria and severity of pulmonary fibrosis: analysis of 52 patients. Ultraschall Med. 2016; 37:379–85.16. Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound. 2011; 9:6.
Article17. Lichtenstein D. Whole body ultrasonography in the critically ill. Heidelberg, Berlin, New York: Springer-Verlag;2010.18. Brusasco C, Santori G, Bruzzo E, Trò R, Robba C, Tavazzi G, et al. Quantitative lung ultrasonography: a putative new algorithm for automatic detection and quantification of B-lines. Crit Care. 2019; 23:288.
Article19. Lichtenstein D, Mezière G, Biderman P, Gepner A. The "lung point": an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000; 26:1434–40.
Article20. De Luca C, Valentino M, Rimondi MR, Branchini M, Baleni MC, Barozzi L. Use of chest sonography in acute-care radiology. J Ultrasound. 2008; 11:125–34.
Article21. Murphy M, Nagdev A, Sisson C. Lack of lung sliding on ultrasound does not always indicate a pneumothorax. Resuscitation. 2008; 77:270.
Article22. Ball CG, Kirkpatrick AW, Feliciano DV. The occult pneumothorax: what have we learned? Can J Surg. 2009; 52:E173–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prenatal Diagnosis and Outcome of Congenital Lung Mass
- Fracture Detection with Ultrasonography in Multiple Bony Structures
- Role of ultrasound in the evaluation of first-trimester pregnancies in the acute setting
- Lung Ultrasound (in the Critically Ill) Superior to CT: the Example of Lung Sliding
- Pediatric lung ultrasound: its role in the perioperative period