Int J Stem Cells.
2023 Feb;16(1):117-122. 10.15283/ijsc22125.
Reduced Cytotoxicity by Repetitive mRNA Transfection in Differentiated Neurons
- Affiliations
-
- 1Graduate School of Biomedical Science and Engineering, Hanyang University, Seoul, Korea
- 2Hanyang Biomedical Research Institute, Hanyang University, Seoul, Korea
- 3Neuroregeneration and Stem Cell Programs, Institute for Cell Engineering, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 4Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
- 5Department of Microbiology, College of Medicine, Hanyang University, Seoul, Korea
- KMID: 2539626
- DOI: http://doi.org/10.15283/ijsc22125
Abstract
- Background and Objectives
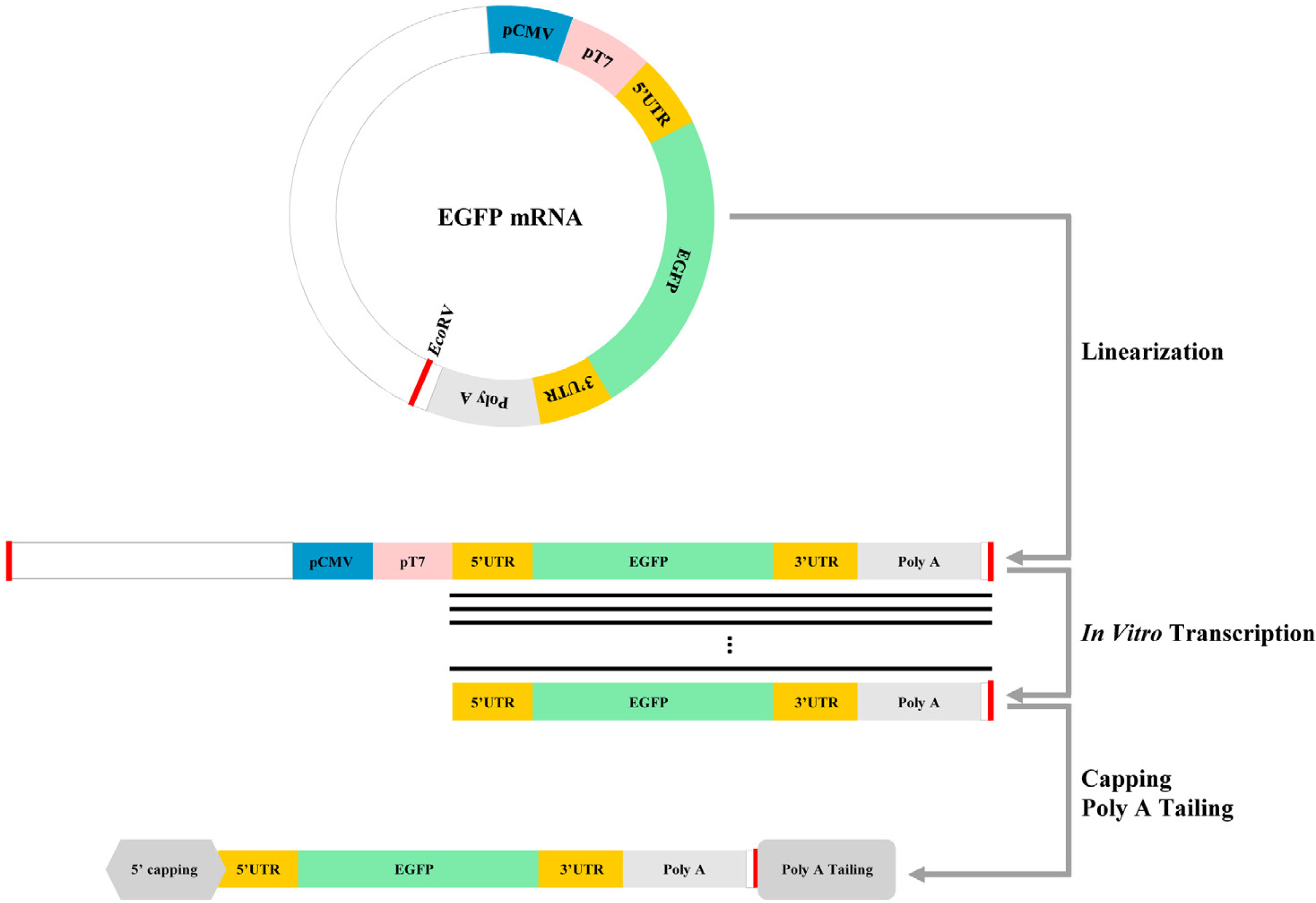
mRNA-based protein expression technology has been used to express functional proteins. We have previously generated dopamine neurons from rat-embryo derived neural precursor cells (NPCs) through repeated transfection of synthetic transcription factor mRNA encoding dopamine-inducible genes. However, NPCs began to die approximately 10 d post-transfection. In this study, we examined a long-term transfection protocol that did not affect cell viability.
Methods and Results
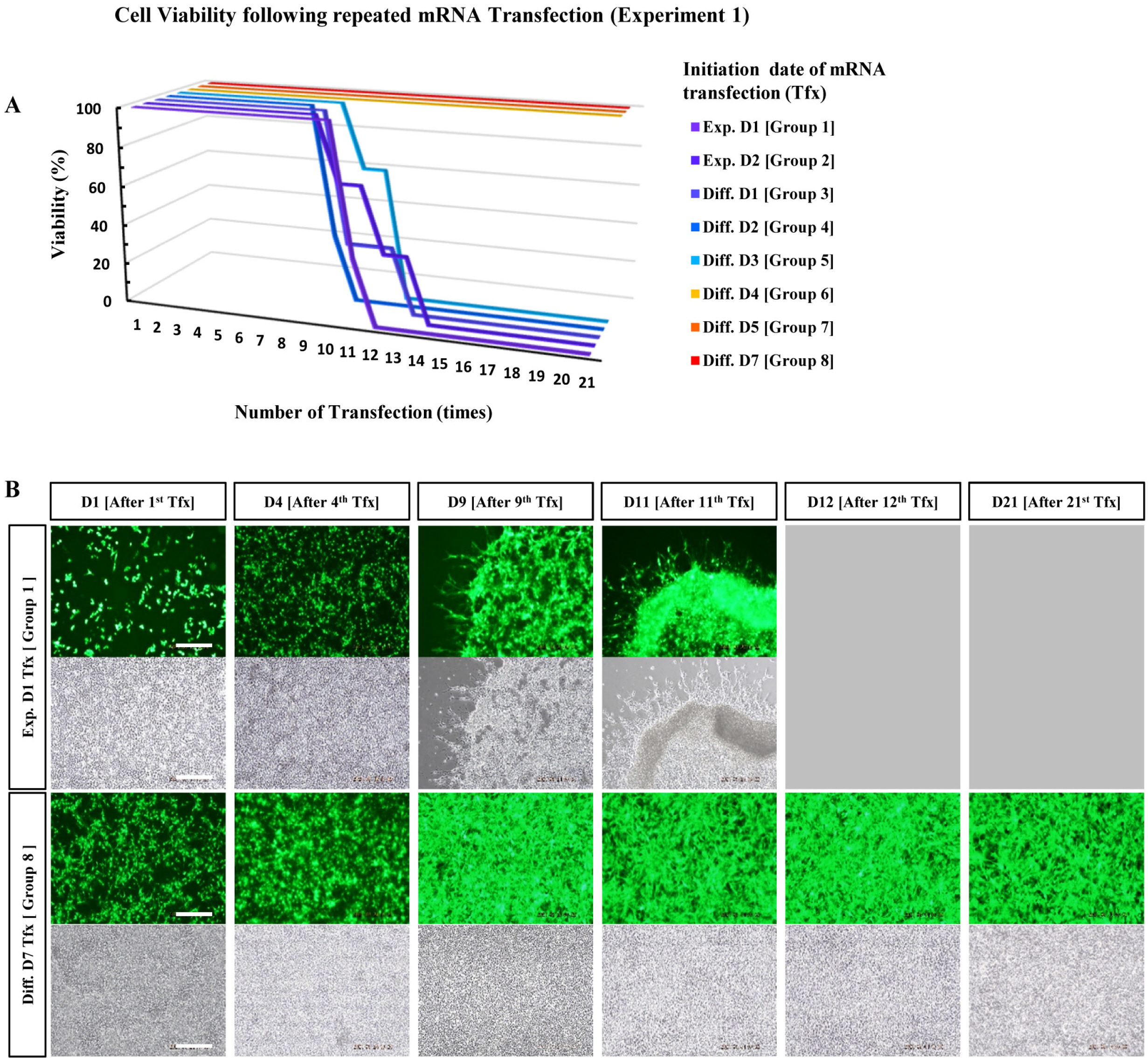
Experiments were performed in eight groups sorted according to the start date of mRNA transfection. mRNA was transfected into NPCs daily for 21 d and live cell images of each group were recorded. NPCs which were differentiated for more than five days showed sustained gene expression and appreciable viability despite daily mRNA transfection for 21 d.
Conclusions
Repeated mRNA transfection requires cells with a sufficient differentiation period.
Figure
Reference
-
References
1. Sung YK, Kim SW. 2019; Recent advances in the development of gene delivery systems. Biomater Res. 23:8. DOI: 10.1186/s40824-019-0156-z. PMID: 30915230. PMCID: PMC6417261. PMID: bf6a6ce2d576497899d4a5004c821aee.2. Sahin U, Karikó K, Türeci Ö. 2014; mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 13:759–780. DOI: 10.1038/nrd4278. PMID: 25233993.3. McLenachan S, Zhang D, Palomo AB, Edel MJ, Chen FK. 2013; mRNA transfection of mouse and human neural stem cell cultures. PLoS One. 8:e83596. DOI: 10.1371/journal.pone.0083596. PMID: 24386231. PMCID: PMC3873397. PMID: 691578f40808496b910b2f0f0c0b4bc2.4. Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, Gupta D, McPherson J, Malwadkar P, Gupta M, Bell B, Doi A, Jung N, Li X, Lynes MS, Brookes E, Cherry AB, Demirbas D, Tsankov AM, Zon LI, Rubin LL, Feinberg AP, Meissner A, Cowan CA, Daley GQ. 2015; A comparison of non-integrating reprogramming methods. Nat Biotechnol. 33:58–63. DOI: 10.1038/nbt.3070. PMID: 25437882. PMCID: PMC4329913.5. Yamamoto A, Kormann M, Rosenecker J, Rudolph C. 2009; Current prospects for mRNA gene delivery. Eur J Pharm Biopharm. 71:484–489. DOI: 10.1016/j.ejpb.2008.09.016. PMID: 18948192.6. Mandal PK, Rossi DJ. 2013; Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc. 8:568–582. DOI: 10.1038/nprot.2013.019. PMID: 23429718.7. Kim SM, Lim MS, Lee EH, Jung SJ, Chung HY, Kim CH, Park CH. 2017; Efficient generation of dopamine neurons by synthetic transcription factor mRNAs. Mol Ther. 25:2028–2037. DOI: 10.1016/j.ymthe.2017.06.015. PMID: 28705346. PMCID: PMC5589083.8. Bulaklak K, Gersbach CA. 2020; The once and future gene therapy. Nat Commun. 11:5820. DOI: 10.1038/s41467-020-19505-2. PMID: 33199717. PMCID: PMC7670458. PMID: e73be1499a3f43d1abd476b0fd01fb8c.9. Giamas G, Gagliano T. 2022; Cancer gene therapy 2020: highlights from a challenging year. Cancer Gene Ther. 29:1–3. DOI: 10.1038/s41417-021-00340-6. PMID: 33963297. PMCID: PMC8103066.10. Papanikolaou E, Bosio A. 2021; The promise and the hope of gene therapy. Front Genome Ed. 3:618346. DOI: 10.3389/fgeed.2021.618346. PMID: 34713249. PMCID: PMC8525363. PMID: 0150aea3236049339e98244cab643a0d.11. Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. 2021; Viral vector platforms within the gene therapy landscape. Signal Trans-duct Target Ther. 6:53. DOI: 10.1038/s41392-021-00487-6. PMID: 33558455. PMCID: PMC7868676. PMID: 63666028eb554ff786d8586456529231.12. Lundstrom K. 2018; Viral vectors in gene therapy. Diseases. 6:42. DOI: 10.3390/diseases6020042. PMID: 29883422. PMCID: PMC6023384.13. Wang D, Tai PWL, Gao G. 2019; Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 18:358–378. DOI: 10.1038/s41573-019-0012-9. PMID: 30710128. PMCID: PMC6927556.14. Huang CL, Leblond AL, Turner EC, Kumar AH, Martin K, Whelan D, O'Sullivan DM, Caplice NM. 2015; Synthetic chemically modified mrna-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol Pharm. 12:991–996. DOI: 10.1021/mp5006239. PMID: 25588055.15. Zhou X, Hao R, Chen C, Su Z, Zhao L, Luo Z, Xie W. 2020; Rapid delivery of nanobodies/VHHs into living cells via expressing in vitro-transcribed mRNA. Mol Ther Methods Clin Dev. 17:401–408. DOI: 10.1016/j.omtm.2020.01.008. PMID: 32128345. PMCID: PMC7044678.16. Wang XF, Cynader MS. 1999; Effects of astrocytes on neuronal attachment and survival shown in a serum-free co-culture system. Brain Res Brain Res Protoc. 4:209–216. DOI: 10.1016/S1385-299X(99)00019-7. PMID: 10446416.17. Chang MY, Son H, Lee YS, Lee SH. 2003; Neurons and astrocytes secrete factors that cause stem cells to differentiate into neurons and astrocytes, respectively. Mol Cell Neurosci. 23:414–426. DOI: 10.1016/S1044-7431(03)00068-X. PMID: 12837625.18. Wang FW, Hao HB, Zhao SD, Zhang YM, Liu Q, Liu HJ, Liu SM, Yuan QH, Bing LJ, Ling EA, Hao AJ. 2011; Roles of activated astrocyte in neural stem cell proliferation and differentiation. Stem Cell Res. 7:41–53. DOI: 10.1016/j.scr.2011.03.004. PMID: 21530437.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NELL2 gene as regulator of cell cycle in neuron differentiation
- Distribution of Neuropeptide mRNA-Containing Neurons and Changes of Their Gene Expression in the Rat Periaqueductal Gray in a Neuropathic Pain Model
- Apoptosis of Prostate Cancer by Inhibition of Bcl-xL Expression
- The Effects of Psychotropic Drugs on the beta-Amyloid-Induced Cytotoxicity in PC12 Cells
- Molecular Pathogenesis and Targeted Therapies in Well-Differentiated Thyroid Carcinoma