Int J Stem Cells.
2023 Feb;16(1):93-107. 10.15283/ijsc21204.
Tracking of Stem Cells from Human Exfoliated Deciduous Teeth Labeled with Molday ION Rhodamine-B during Periodontal Bone Regeneration in Rats
- Affiliations
-
- 1The Institute for Tissue Engineering and Regenerative Medicine, Liaocheng People’s Hospital, Liaocheng, China
- 2Department of MRI, Liaocheng People’s Hospital, Liaocheng, China
- 3Department of Stomatology, Liaocheng People’s Hospital, Liaocheng, China
- 4Department of Orthopedics, Liaocheng People’s Hospital, Liaocheng, China
- 5The Translational Research Laboratory of Stem Cells and Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- KMID: 2539624
- DOI: http://doi.org/10.15283/ijsc21204
Abstract
- Background and Objectives
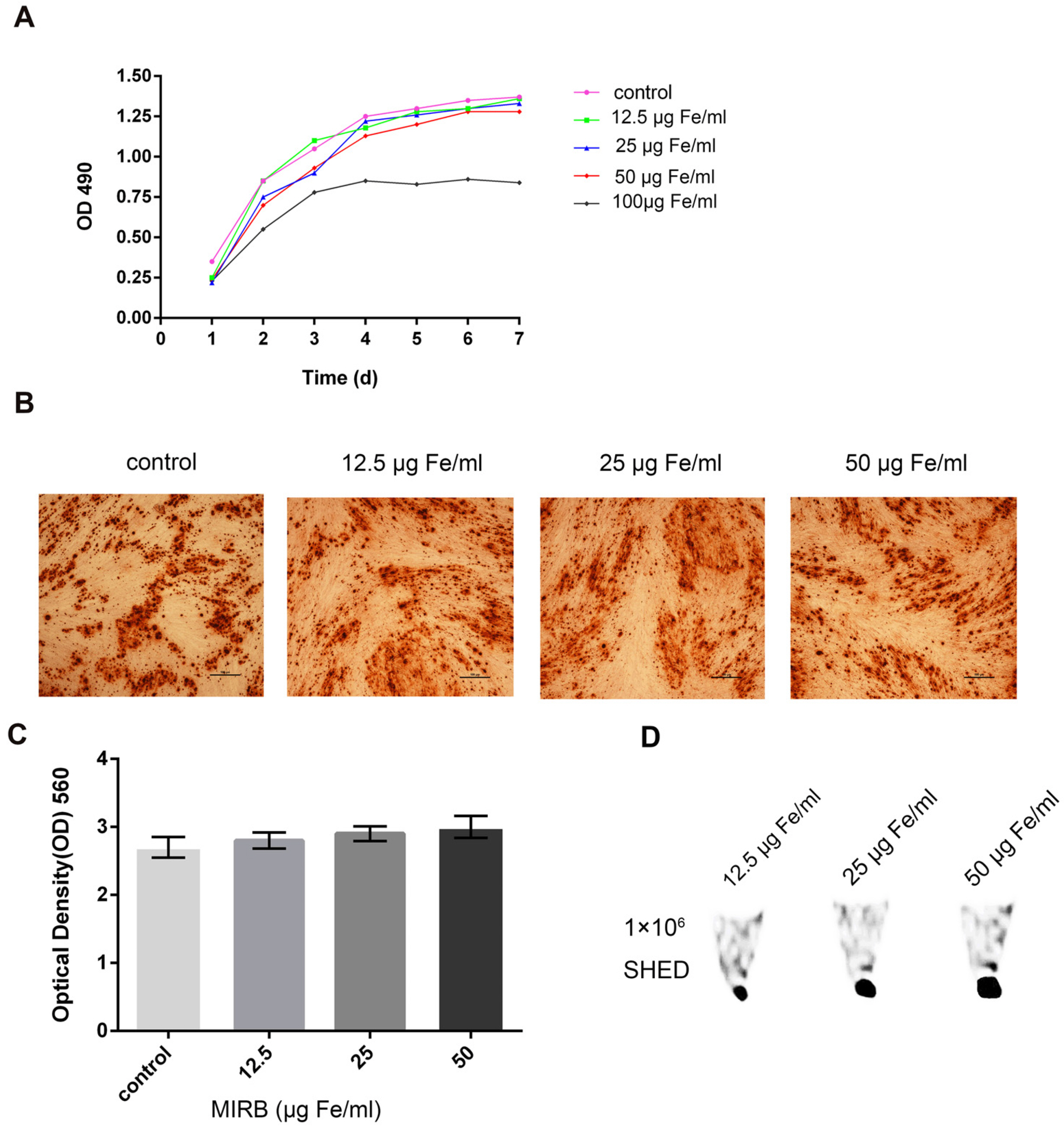
Chronic periodontitis can lead to alveolar bone resorption and eventually tooth loss. Stem cells from exfoliated deciduous teeth (SHED) are appropriate bone regeneration seed cells. To track the survival, migration, and differentiation of the transplanted SHED, we used super paramagnetic iron oxide particles (SPIO) Molday ION Rhodamine-B (MIRB) to label and monitor the transplanted cells while repairing periodontal bone defects.
Methods and Results
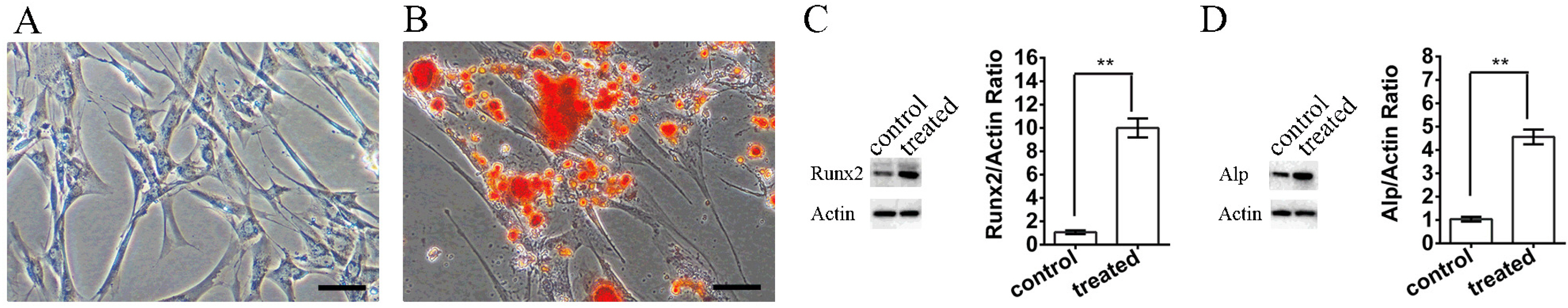
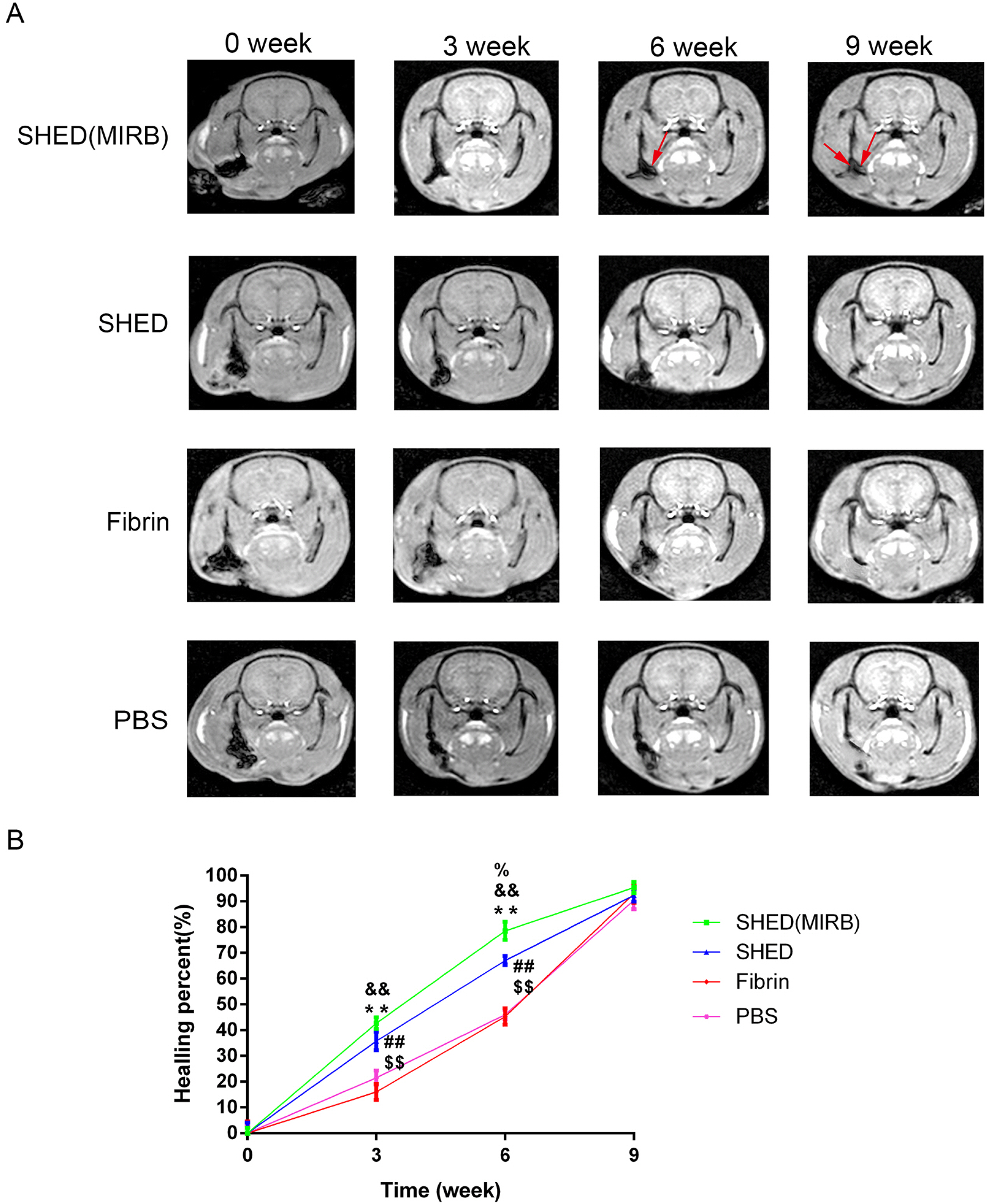
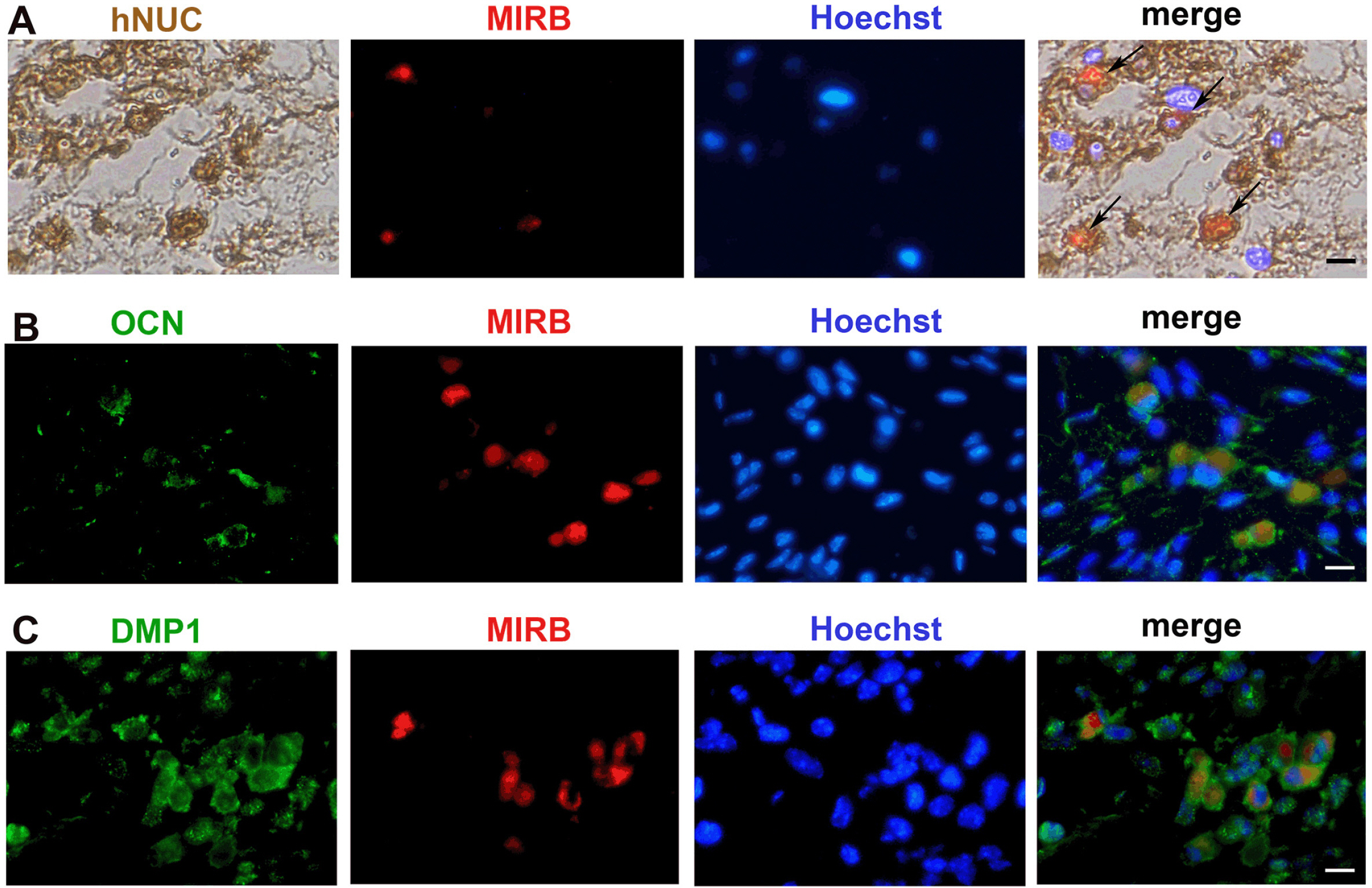
We determined an appropriate dose of MIRB for labeling SHED by examining the growth and osteogenic differentiation of labeled SHED. Finally, SHED was labeled with 25 μg Fe/ml MIRB before being transplanted into rats. Magnetic resonance imaging was used to track SHED survival and migration in vivo due to a low-intensity signal artifact caused by MIRB. HE and immunohistochemical analyses revealed that both MIRB-labeled and unlabeled SHED could promote periodontal bone regeneration. The colocalization of hNUC and MIRB demonstrated that SHED transplanted into rats could survive in vivo. Furthermore, some MIRB-positive cells expressed the osteoblast and osteocyte markers OCN and DMP1, respectively. Enzyme-linked immunosorbent assay revealed that SHED could secrete protein factors, such as IGF-1, OCN, ALP, IL-4, VEGF, and bFGF, which promote bone regeneration. Immunofluorescence staining revealed that the transplanted SHED was surrounded by a large number of host-derived Runx2- and Col II-positive cells that played important roles in the bone healing process.
Conclusions
SHED could promote periodontal bone regeneration in rats, and the survival of SHED could be tracked in vivo by labeling them with MIRB. SHED are likely to promote bone healing through both direct differentiation and paracrine mechanisms.
Keyword
Figure
Reference
-
References
1. Van Dyke TE. 2017; Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. 58:21–36. DOI: 10.1016/j.mam.2017.04.006. PMID: 28483532. PMCID: PMC5660638.2. Hajishengallis G, Chavakis T, Lambris JD. 2020; Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol 2000. 84:14–34. DOI: 10.1111/prd.12331. PMID: 32844416. PMCID: PMC7457922.3. Qiu J, Wang X, Zhou H, Zhang C, Wang Y, Huang J, Liu M, Yang P, Song A. 2020; Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res Ther. 11:42. DOI: 10.1186/s13287-019-1546-9. PMID: 32014015. PMCID: PMC6998241. PMID: 07974615cc674308b98377718edb3909.4. Nuñez J, Vignoletti F, Caffesse RG, Sanz M. 2019; Cellular therapy in periodontal regeneration. Periodontol 2000. 79:107–116. DOI: 10.1111/prd.12250. PMID: 30892768.5. Sordi MB, Curtarelli RB, Mantovani IF, Moreira AC, Fernandes CP, Cruz ACC, Magini RS. 2022; Enhanced osteoinductive capacity of poly(lactic-co-glycolic) acid and biphasic ceramic scaffolds by embedding simvastatin. Clin Oral Investig. 26:2693–2701. DOI: 10.1007/s00784-021-04240-9. PMID: 34694495.6. Subhi H, Husein A, Mohamad D, Nik Abdul Ghani NR, Nurul AA. 2021; Chitosan-based accelerated Portland cement promotes dentinogenic/osteogenic differentiation and mineralization activity of SHED. Polymers (Basel). 13:3358. DOI: 10.3390/polym13193358. PMID: 34641172. PMCID: PMC8512062. PMID: c2d796c62b234389884801a9df9ea586.7. Zaw SYM, Kaneko T, Zaw ZCT, Sone PP, Murano H, Gu B, Okada Y, Han P, Katsube KI, Okiji T. 2020; Crosstalk between dental pulp stem cells and endothelial cells augments angiogenic factor expression. Oral Dis. 26:1275–1283. DOI: 10.1111/odi.13341. PMID: 32248596.8. da Silva AAF, Rinco UGR, Jacob RGM, Sakai VT, Mariano RC. 2022; The effectiveness of hydroxyapatite-beta tricalcium phosphate incorporated into stem cells from human exfoliated deciduous teeth for reconstruction of rat calvarial bone defects. Clin Oral Investig. 26:595–608. DOI: 10.1007/s00784-021-04038-9. PMID: 34169375.9. Novais A, Lesieur J, Sadoine J, Slimani L, Baroukh B, Saubaméa B, Schmitt A, Vital S, Poliard A, Hélary C, Rochefort GY, Chaussain C, Gorin C. 2019; Priming dental pulp stem cells from human exfoliated deciduous teeth with fibroblast growth factor-2 enhances mineralization within tissue-engineered constructs implanted in craniofacial bone defects. Stem Cells Transl Med. 8:844–857. DOI: 10.1002/sctm.18-0182. PMID: 31016898. PMCID: PMC6646701.10. Lee JM, Kim HY, Park JS, Lee DJ, Zhang S, Green DW, Okano T, Hong JH, Jung HS. 2019; Developing palatal bone using human mesenchymal stem cell and stem cells from exfoliated deciduous teeth cell sheets. J Tissue Eng Regen Med. 13:319–327. DOI: 10.1002/term.2811. PMID: 30644640.11. Han Y, Zhang L, Zhang C, Dissanayaka WL. 2021; Guiding lineage specific differentiation of SHED for target tissue/organ regeneration. Curr Stem Cell Res Ther. 16:518–534. DOI: 10.2174/1574888X15666200929125840. PMID: 32990539.12. Makela AV, Murrell DH, Parkins KM, Kara J, Gaudet JM, Foster PJ. 2016; Cellular imaging with MRI. Top Magn Reson Imaging. 25:177–186. DOI: 10.1097/RMR.0000000000000101. PMID: 27748707.13. Peng C, Yang K, Xiang P, Zhang C, Zou L, Wu X, Gao Y, Kang Z, He K, Liu J, Cheng M, Wang J, Chen L. 2013; Effect of transplantation with autologous bone marrow stem cells on acute myocardial infarction. Int J Cardiol. 162:158–165. DOI: 10.1016/j.ijcard.2011.05.077. PMID: 21640407.14. Jin WN, Yang X, Li Z, Li M, Shi SX, Wood K, Liu Q, Fu Y, Han W, Xu Y, Shi FD, Liu Q. 2016; Non-invasive tracking of CD4+ T cells with a paramagnetic and fluorescent nanoparticle in brain ischemia. J Cereb Blood Flow Metab. 36:1464–1476. DOI: 10.1177/0271678X15611137. PMID: 26661207. PMCID: PMC4971610.15. He T, Sun S. 2022; Evaluation of the therapeutic efficacy of human bone marrow mesenchymal stem cells with COX-2 silence and TGF-β3 overexpression in rabbits with antigen-induced arthritis. Exp Cell Res. 410:112945. DOI: 10.1016/j.yexcr.2021.112945. PMID: 34838812.16. Ma L, Li MW, Bai Y, Guo HH, Wang SC, Yu Q. 2017; Biological characteristics of fluorescent superparamagnetic iron oxide labeled human dental pulp stem cells. Stem Cells Int. 2017:4837503. DOI: 10.1155/2017/4837503. PMID: 28298928. PMCID: PMC5337366.17. Zhang N, Chen B, Wang W, Chen C, Kang J, Deng SQ, Zhang B, Liu S, Han F. 2016; Isolation, characterization and multi-lineage differentiation of stem cells from human exfoliated deciduous teeth. Mol Med Rep. 14:95–102. DOI: 10.3892/mmr.2016.5214. PMID: 27151462. PMCID: PMC4918624.18. Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, Ivanovski S. 2018; Tissue engineered constructs for periodontal regeneration: current status and future perspectives. Adv Healthc Mater. 7:e1800457. DOI: 10.1002/adhm.201800457. PMID: 30146758.19. Huang H, Du X, He Z, Yan Z, Han W. 2021; Nanoparticles for stem cell tracking and the potential treatment of cardiovascular diseases. Front Cell Dev Biol. 9:662406. DOI: 10.3389/fcell.2021.662406. PMID: 34277609. PMCID: PMC8283769. PMID: 6b92e1d57bd5445cbd5997b492414424.20. Moonshi SS, Wu Y, Ta HT. 2022; Visualizing stem cells in vivo using magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 14:e1760. DOI: 10.1002/wnan.1760. PMID: 34651465.21. Dabrowska S, Del Fattore A, Karnas E, Frontczak-Baniewicz M, Kozlowska H, Muraca M, Janowski M, Lukomska B. 2018; Imaging of extracellular vesicles derived from human bone marrow mesenchymal stem cells using fluorescent and magnetic labels. Int J Nanomedicine. 13:1653–1664. DOI: 10.2147/IJN.S159404. PMID: 29593411. PMCID: PMC5865569.22. Weissleder R. 2001; A clearer vision for in vivo imaging. Nat Biotechnol. 19:316–317. DOI: 10.1038/86684. PMID: 11283581.23. Hariharan A, Iyer J, Wang A, Tran SD. 2021; Tracking of oral and craniofacial stem cells in tissue development, regeneration, and diseases. Curr Osteoporos Rep. 19:656–668. DOI: 10.1007/s11914-021-00705-8. PMID: 34741728.24. Burk J, Berner D, Brehm W, Hillmann A, Horstmeier C, Josten C, Paebst F, Rossi G, Schubert S, Ahrberg AB. 2016; Long-term cell tracking following local injection of mesenchymal stromal cells in the equine model of induced tendon disease. Cell Transplant. 25:2199–2211. DOI: 10.3727/096368916X692104. PMID: 27392888.25. Zhang S, Xie D, Zhang Q. 2021; Mesenchymal stem cells plus bone repair materials as a therapeutic strategy for abnormal bone metabolism: evidence of clinical efficacy and mechanisms of action implied. Pharmacol Res. 172:105851. DOI: 10.1016/j.phrs.2021.105851. PMID: 34450314.26. Collignon AM, Castillo-Dali G, Gomez E, Guilbert T, Lesieur J, Nicoletti A, Acuna-Mendoza S, Letourneur D, Chaussain C, Rochefort GY, Poliard A. 2019; Mouse Wnt1-CRE-RosaTomato dental pulp stem cells directly contribute to the calvarial bone regeneration process. Stem Cells. 37:701–711. DOI: 10.1002/stem.2973. PMID: 30674073.27. Motoike S, Kajiya M, Komatsu N, Horikoshi S, Ogawa T, Sone H, Matsuda S, Ouhara K, Iwata T, Mizuno N, Fujita T, Ikeya M, Kurihara H. 2019; Clumps of mesenchymal stem cell/extracellular matrix complexes generated with xeno-free conditions facilitate bone regeneration via direct and indirect osteogenesis. Int J Mol Sci. 20:3970. DOI: 10.3390/ijms20163970. PMID: 31443173. PMCID: PMC6720767.28. Takarada T, Nakazato R, Tsuchikane A, Fujikawa K, Iezaki T, Yoneda Y, Hinoi E. 2016; Genetic analysis of Runx2 function during intramembranous ossification. Development. 143:211–218. DOI: 10.1242/dev.128793. PMID: 26657773.29. Kozhemyakina E, Lassar AB, Zelzer E. 2015; A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 142:817–831. DOI: 10.1242/dev.105536. PMID: 25715393. PMCID: PMC4352987.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indirect Co-Culture of Stem Cells from Human Exfoliated Deciduous Teeth and Oral Cells in a Microfluidic Platform
- Modulation of osteoblastic/odontoblastic differentiation of adult mesenchymal stem cells through gene introduction: a brief review
- Induced Pluripotent Stem (iPS) Cells in Dentistry: A Review
- Cytotoxicity of Various Calcium Silicate-based Materials with Stem Cells from Deciduous Teeth
- Stem cells from human exfoliated deciduous teeth attenuate trigeminal neuralgia in rats by inhibiting endoplasmic reticulum stress