Korean J Pain.
2022 Oct;35(4):383-390. 10.3344/kjp.2022.35.4.383.
Stem cells from human exfoliated deciduous teeth attenuate trigeminal neuralgia in rats by inhibiting endoplasmic reticulum stress
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, China Medical University, Shenyang, China
- 2Liaoning Provincial Key Laboratory of Oral Diseases, Shenyang, China
- 3Department of Anesthesia, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Painless Dental Treatment Center, Hospital of Stomatology, China Medical University, Shenyang, China
- KMID: 2533978
- DOI: http://doi.org/10.3344/kjp.2022.35.4.383
Abstract
- Background
The treatment of trigeminal neuralgia remains a challenging issue. Stem cells from human exfoliated deciduous teeth (SHED) provide optimized therapy for chronic pain. This study aimed to investigate the mechanisms underlying the attenuation of trigeminal neuralgia by SHED.
Methods
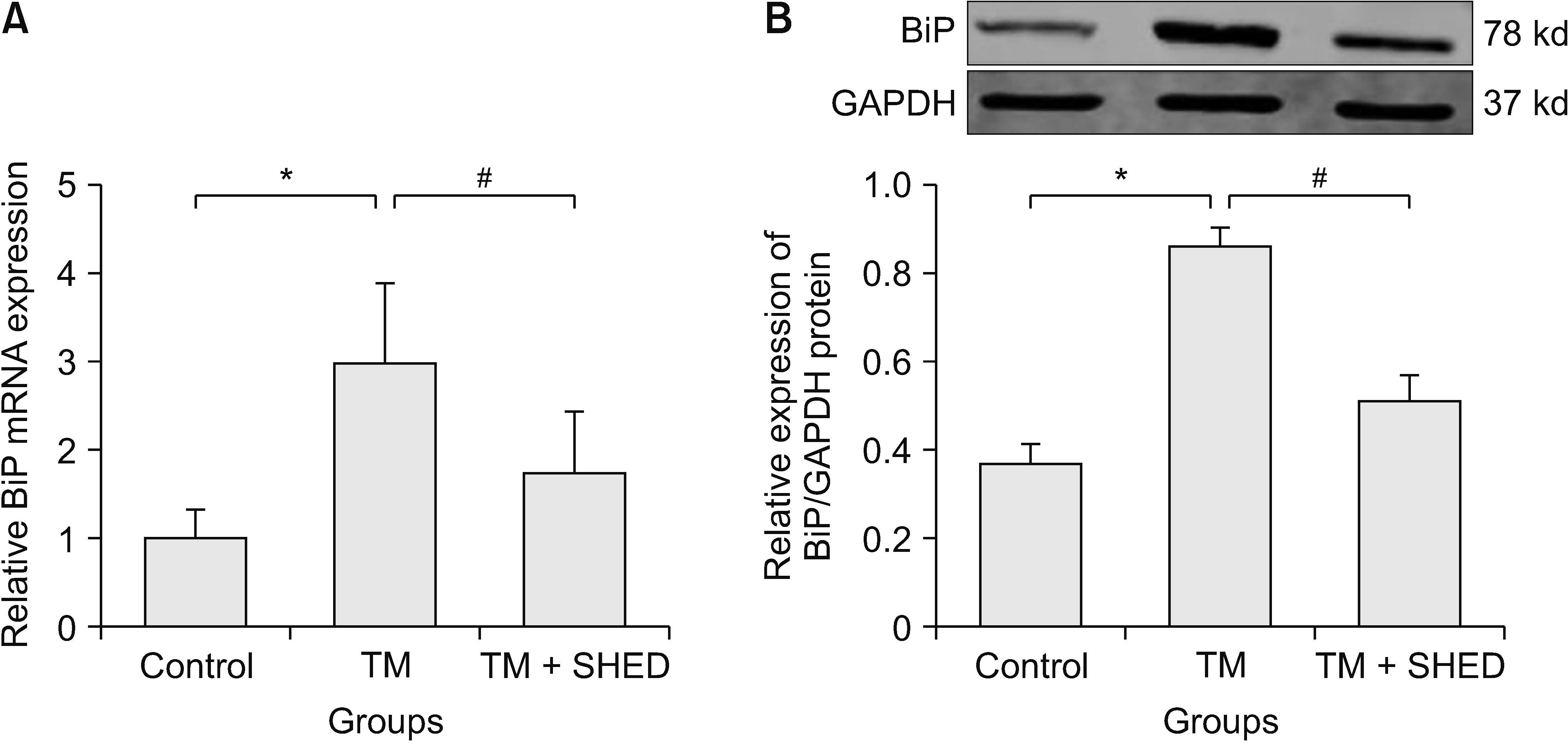
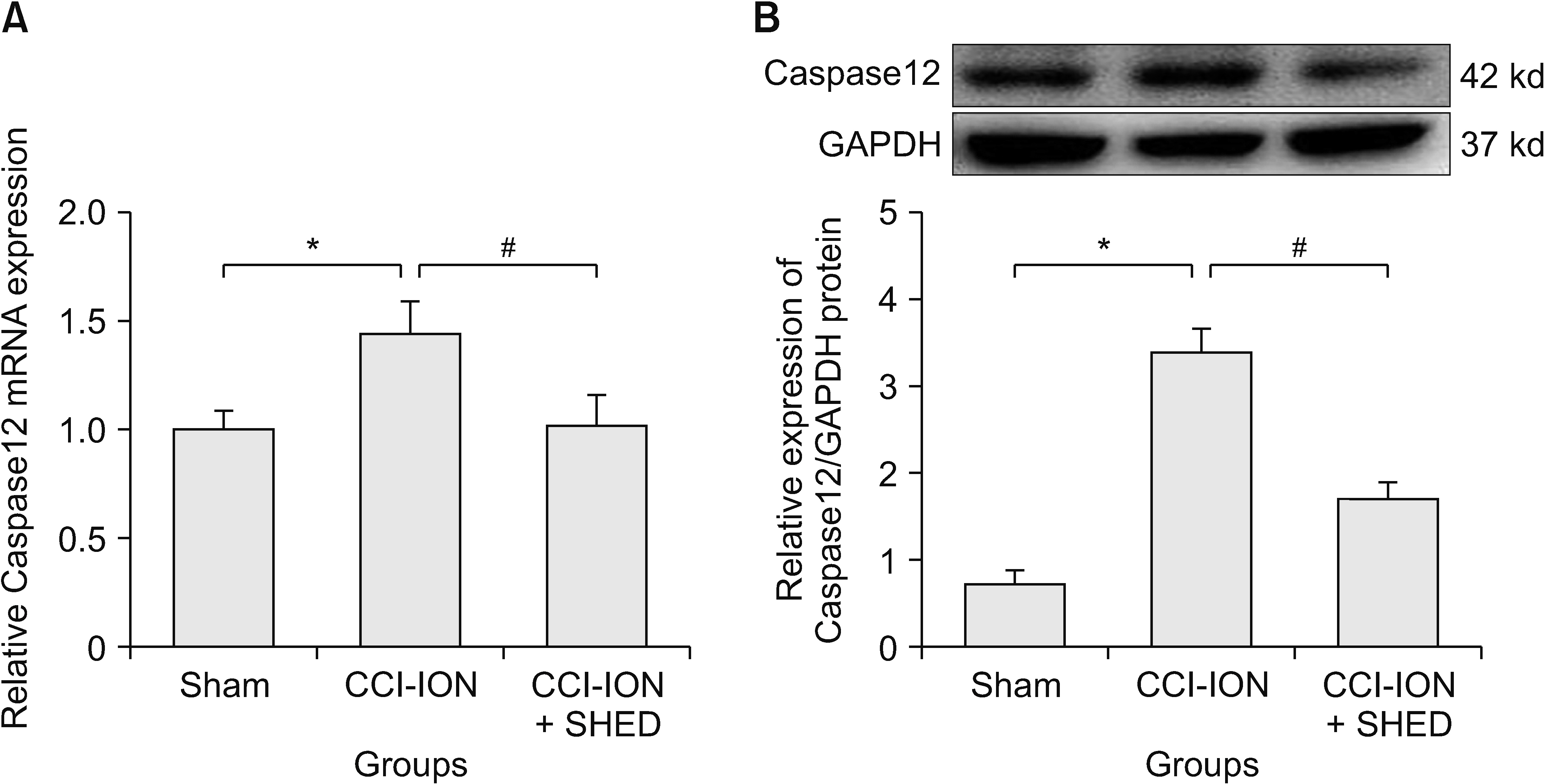
Trigeminal neuralgia was induced by chronic constriction injury of the infraorbital nerve. The mechanical threshold was assessed after model establishment and local SHED transplantation. Endoplasmic reticulum (ER) morphology and Caspase12 expression in trigeminal ganglion (TG) was evaluated as well. BiP expression was observed in PC12 cells induced by tunicamycin.
Results
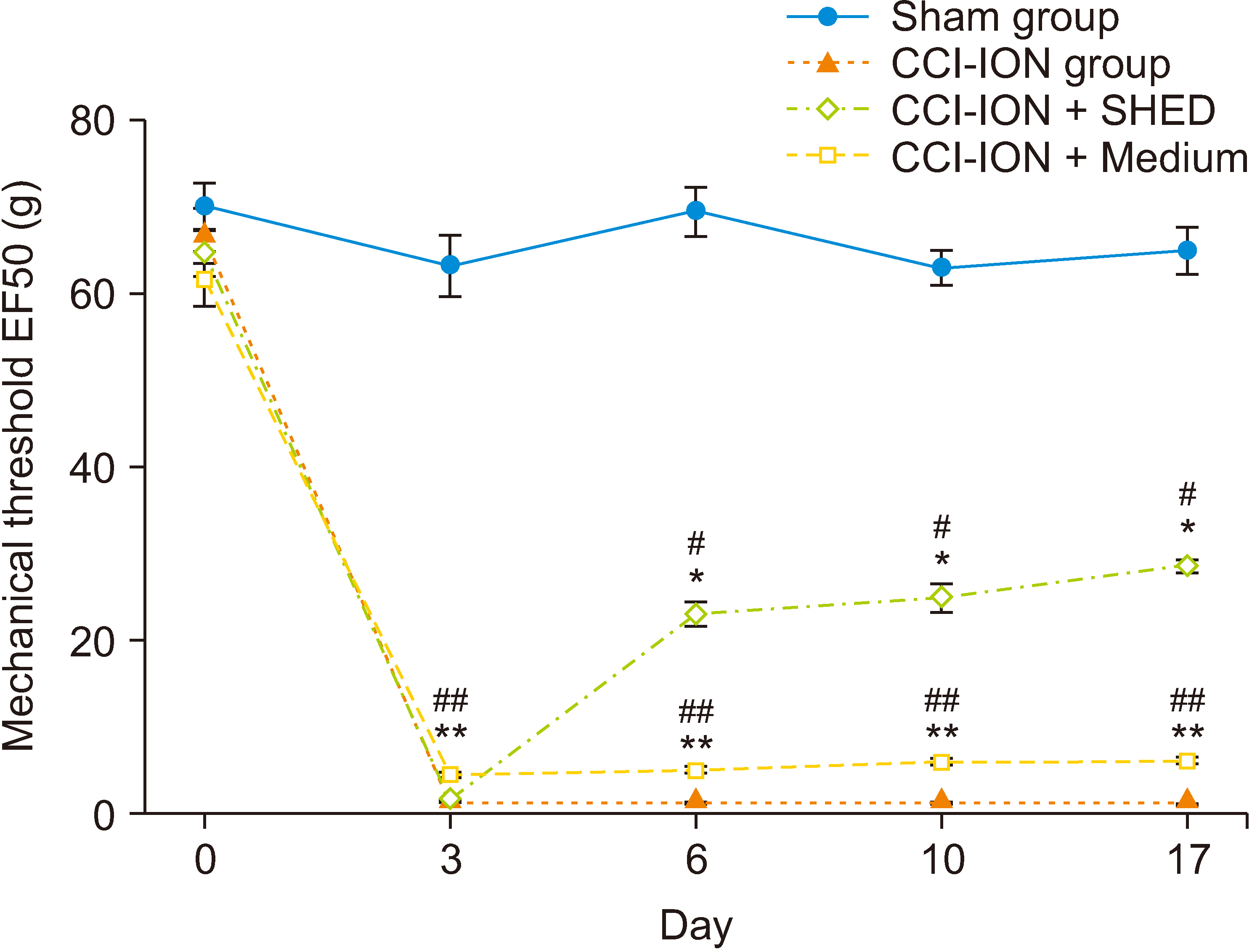
The local transplantation of SHED could relieve trigeminal neuralgia in rats. Further, transmission electron microscopy revealed swelling of the ER in rats with trigeminal neuralgia. Moreover, SHED inhibited the tunicamycin-induced up-regulated expression of BiP mRNA and protein in vitro. Additionally, SHED decreased the up-regulated expression of Caspase12 mRNA and protein in the TG of rats caused by trigeminal neuralgia after chronic constriction injury of the infraorbital nerve mode.
Conclusions
This findings demonstrated that SHED could alleviate pain by relieving ER stress which provide potential basic evidence for clinical pain treatment.
Keyword
Figure
Reference
-
1. Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T, et al. 2020; Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 19:784–96. DOI: 10.1016/S1474-4422(20)30233-7. PMID: 32822636. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089515336&origin=inward.
Article2. Anoop M, Datta I. 2021; Stem cells derived from human exfoliated deciduous teeth (SHED) in neuronal disorders: a review. Curr Stem Cell Res Ther. 16:535–50. DOI: 10.2174/1574888X16666201221151512. PMID: 33349220. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85113789414&origin=inward.
Article3. Fuloria S, Jain A, Singh S, Hazarika I, Salile S, Fuloria NK. 2021; Regenerative potential of stem cells derived from human exfoliated deciduous (SHED) teeth during engineering of human body tissues. Curr Stem Cell Res Ther. 16:507–17. DOI: 10.2174/1574888X16999201231213206. PMID: 33390148. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85114056149&origin=inward.
Article4. Fayazi N, Sheykhhasan M, Soleimani Asl S, Najafi R. 2021; Stem cell-derived exosomes: a new strategy of neurodegenerative disease treatment. Mol Neurobiol. 58:3494–514. DOI: 10.1007/s12035-021-02324-x. PMID: 33745116. PMCID: PMC7981389. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85103188198&origin=inward.
Article5. Kunimatsu R, Nakajima K, Awada T, Tsuka Y, Abe T, Ando K, et al. 2018; Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 501:193–8. DOI: 10.1016/j.bbrc.2018.04.213. PMID: 29730288. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046699495&origin=inward.
Article6. Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. 2009; Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 35:1536–42. DOI: 10.1016/j.joen.2009.07.024. PMID: 19840643. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70349973455&origin=inward.
Article7. Bai X, Zhang X, Wang C, Liu Y, Liu X, Fan Y, et al. 2021; Stem cells from human exfoliated deciduous teeth attenuate trigeminal neuralgia in rats. Stem Cells Int. 2021:8819884. DOI: 10.1155/2021/8819884. PMID: 33531911. PMCID: PMC7834821. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100570726&origin=inward.
Article8. Bettigole SE, Glimcher LH. 2015; Endoplasmic reticulum stress in immunity. Annu Rev Immunol. 33:107–38. DOI: 10.1146/annurev-immunol-032414-112116. PMID: 25493331. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84927636122&origin=inward.
Article9. Zhang D, Zhou Q, Huang D, He L, Zhang H, Hu B, et al. 2019; ROS/JNK/c-Jun axis is involved in oridonin-induced caspase-dependent apoptosis in human colorectal cancer cells. Biochem Biophys Res Commun. 513:594–601. DOI: 10.1016/j.bbrc.2019.04.011. PMID: 30981511. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064059670&origin=inward.
Article10. Jin H, Komita M, Aoe T. 2017; The role of BiP retrieval by the KDEL receptor in the early secretory pathway and its effect on protein quality control and neurodegeneration. Front Mol Neurosci. 10:222. DOI: 10.3389/fnmol.2017.00222. PMID: 28769758. PMCID: PMC5511815. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026666513&origin=inward.
Article11. Wu M, Liu X, Li Z, Huang X, Guo H, Guo X, et al. 2021; SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif. 54:e13074. DOI: 10.1111/cpr.13074. PMID: 34101281. PMCID: PMC8249784. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85107328671&origin=inward.
Article12. Krzyzanowska A, Pittolo S, Cabrerizo M, Sánchez-López J, Krishnasamy S, Venero C, et al. 2011; Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. J Neurosci Methods. 201:46–54. DOI: 10.1016/j.jneumeth.2011.07.006. PMID: 21782847. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80052317834&origin=inward.
Article13. Bai X, Xiao K, Yang Z, Zhang Z, Li J, Yan Z, et al. 2022; Stem cells from human exfoliated deciduous teeth relieve pain via downregulation of c-Jun in a rat model of trigeminal neuralgia. J Oral Rehabil. 49:219–27. DOI: 10.1111/joor.13243. PMID: 34386989. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85112710415&origin=inward.
Article14. Shetty AK, Bates A. 2016; Potential of GABA-ergic cell therapy for schizophrenia, neuropathic pain, and Alzheimer's and Parkinson's diseases. Brain Res. 1638(Pt A):74–87. DOI: 10.1016/j.brainres.2015.09.019. PMID: 26423935. PMCID: PMC5313260. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84951202711&origin=inward.
Article15. Han YH, Kim KH, Abdi S, Kim TK. 2019; Stem cell therapy in pain medicine. Korean J Pain. 32:245–55. DOI: 10.3344/kjp.2019.32.4.245. PMID: 31569916. PMCID: PMC6813895. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85074338727&origin=inward.
Article16. Guo W, Wang H, Zou S, Gu M, Watanabe M, Wei F, et al. 2011; Bone marrow stromal cells produce long-term pain relief in rat models of persistent pain. Stem Cells. 29:1294–303. DOI: 10.1002/stem.667. PMID: 21630378. PMCID: PMC3277433. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79960864273&origin=inward.
Article17. Abrams MB, Dominguez C, Pernold K, Reger R, Wiesenfeld-Hallin Z, Olson L, et al. 2009; Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor Neurol Neurosci. 27:307–21. DOI: 10.3233/RNN-2009-0480. PMID: 19738324. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70349159499&origin=inward.
Article18. Watanabe S, Uchida K, Nakajima H, Matsuo H, Sugita D, Yoshida A, et al. 2015; Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 33:1902–14. DOI: 10.1002/stem.2006. PMID: 25809552. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84929832810&origin=inward.
Article19. Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. 2012; Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 122:80–90. DOI: 10.1172/JCI59251. PMID: 22133879. PMCID: PMC3248299. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84855453812&origin=inward.
Article20. Nicola FC, Rodrigues LP, Crestani T, Quintiliano K, Sanches EF, Willborn S, et al. 2016; Human dental pulp stem cells transplantation combined with treadmill training in rats after traumatic spinal cord injury. Braz J Med Biol Res. 49:e5319. DOI: 10.1590/1414-431x20165319. PMID: 27509306. PMCID: PMC4988478. PMID: 5b3671fd05174e618020465b674c2eb6. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84981351813&origin=inward.
Article21. Hayashi Y, Kato H, Nonaka K, Nakanishi H. 2021; Stem cells from human exfoliated deciduous teeth attenuate mechanical allodynia in mice through distinct from the siglec-9/MCP-1-mediated tissue-repairing mechanism. Sci Rep. 11:20053. DOI: 10.1038/s41598-021-99585-2. PMID: 34625639. PMCID: PMC8501097. PMID: 81169b5e56444b048baab02f84851c29. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85116787082&origin=inward.
Article22. Xie J, Rao N, Zhai Y, Li J, Zhao Y, Ge L, et al. 2019; Therapeutic effects of stem cells from human exfoliated deciduous teeth on diabetic peripheral neuropathy. Diabetol Metab Syndr. 11:38. DOI: 10.1186/s13098-019-0433-y. PMID: 31131042. PMCID: PMC6525430. PMID: 1eed269eada94797b1d53af62e6d0202. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065879132&origin=inward.
Article23. Huang GT, Gronthos S, Shi S. 2009; Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 88:792–806. DOI: 10.1177/0022034509340867. PMID: 19767575. PMCID: PMC2830488. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70349510607&origin=inward.
Article24. Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, et al. 2010; Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 19:1375–83. DOI: 10.1089/scd.2009.0258. PMID: 20131979. PMCID: PMC3073455. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77956449630&origin=inward.
Article25. Wu FL, Liu WY, Van Poucke S, Braddock M, Jin WM, Xiao J, et al. 2016; Targeting endoplasmic reticulum stress in liver disease. Expert Rev Gastroenterol Hepatol. 10:1041–52. DOI: 10.1080/17474124.2016.1179575. PMID: 27093595. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84965050646&origin=inward.
Article26. Zhan X, Qian B, Cao F, Wu W, Yang L, Guan Q, et al. 2015; An Arabidopsis PWI and RRM motif-containing protein is critical for pre-mRNA splicing and ABA responses. Nat Commun. 6:8139. DOI: 10.1038/ncomms9139. PMID: 26404089. PMCID: PMC5514415. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84942739586&origin=inward.
Article27. Yamaguchi Y, Oh-Hashi K, Matsuoka Y, Takemura H, Yamakita S, Matsuda M, et al. 2018; Endoplasmic reticulum stress in the dorsal root ganglion contributes to the development of pain hypersensitivity after nerve injury. Neuroscience. 394:288–99. DOI: 10.1016/j.neuroscience.2018.08.005. PMID: 30482273. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054067852&origin=inward.
Article28. Inceoglu B, Bettaieb A, Trindade da Silva CA, Lee KS, Haj FG, Hammock BD. 2015; Endoplasmic reticulum stress in the peripheral nervous system is a significant driver of neuropathic pain. Proc Natl Acad Sci U S A. 112:9082–7. DOI: 10.1073/pnas.1510137112. PMID: 26150506. PMCID: PMC4517273. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84937711018&origin=inward.
Article29. Van Opdenbosch N, Lamkanfi M. 2019; Caspases in cell death, inflammation, and disease. Immunity. 50:1352–64. DOI: 10.1016/j.immuni.2019.05.020. PMID: 31216460. PMCID: PMC6611727. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85066798798&origin=inward.
Article30. Jia ZL, Cen J, Wang JB, Zhang F, Xia Q, Wang X, et al. 2019; Mechanism of isoniazid-induced hepatotoxicity in zebrafish larvae: activation of ROS-mediated ERS, apoptosis and the Nrf2 pathway. Chemosphere. 227:541–50. DOI: 10.1016/j.chemosphere.2019.04.026. PMID: 31004821. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064457337&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Indirect Co-Culture of Stem Cells from Human Exfoliated Deciduous Teeth and Oral Cells in a Microfluidic Platform
- Endoplasmic Reticulum Stress and Diabetes
- Tracking of Stem Cells from Human Exfoliated Deciduous Teeth Labeled with Molday ION Rhodamine-B during Periodontal Bone Regeneration in Rats
- Optimal Xeno-free Culture Condition for Clinical Grade Stem Cells from Human Exfoliated Deciduous Teeth
- Endoplasmic Reticulum Stress Responses and Apoptosis