Korean J Physiol Pharmacol.
2023 Mar;27(2):167-176. 10.4196/kjpp.2023.27.2.167.
Rehmannioside D mitigates disease progression in rats with experimental-induced diminished ovarian reserve via Forkhead Box O1/KLOTHO axis
- Affiliations
-
- 1Department of Traditional Chinese Medicine, Maternal and Child Health Hospital of Jiangxi Province, Nanchang, Jiangxi 330006, China
- KMID: 2539571
- DOI: http://doi.org/10.4196/kjpp.2023.27.2.167
Abstract
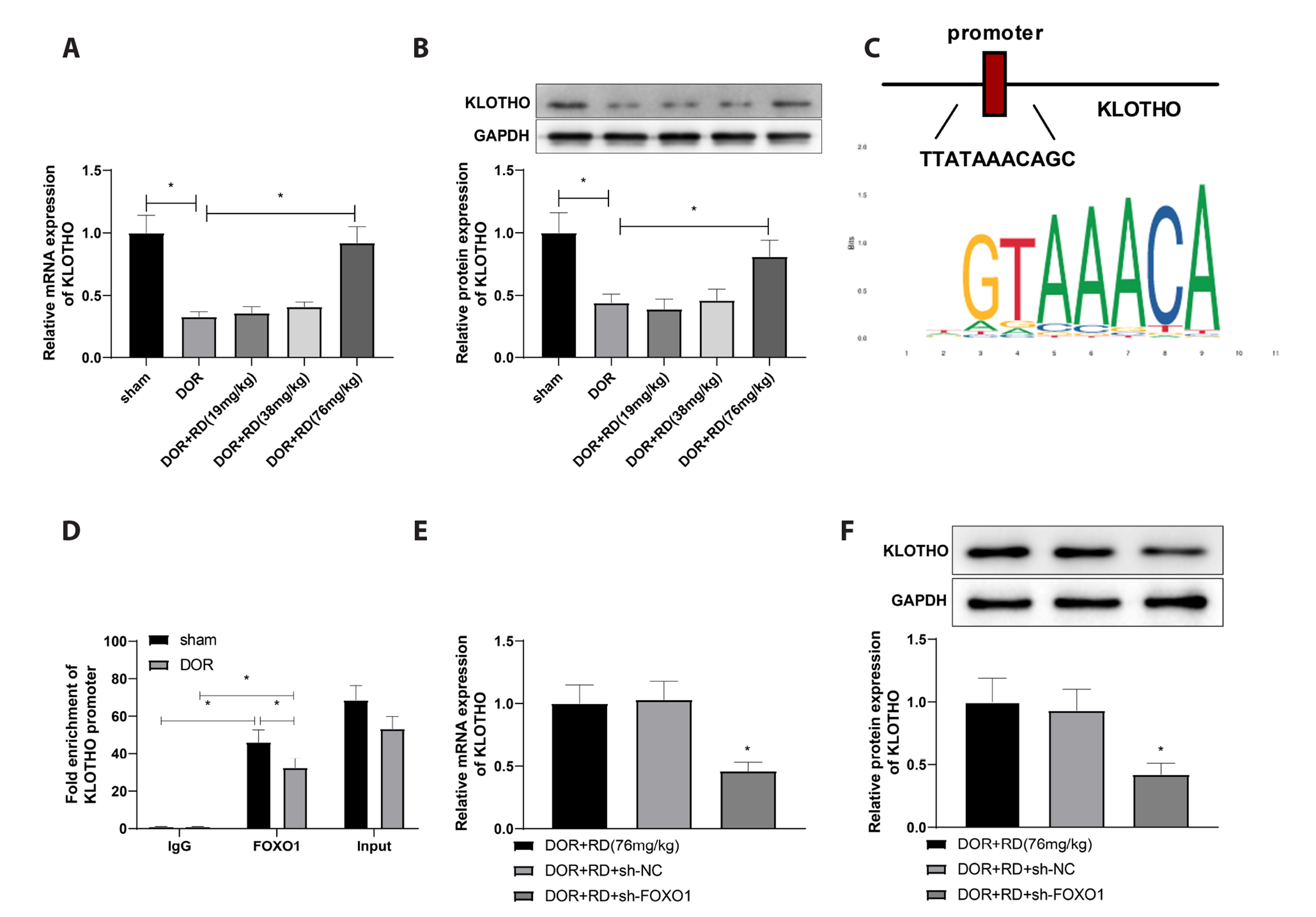
- This study aims to explore the impact of Rehmannioside D (RD) on ovarian functions of rats with diminished ovarian reserve (DOR) and its underlying mechanisms of action. A single injection of cyclophosphamide was performed to establish a DOR rat model, and fourteen days after the injection, the rats were intragastrically administrated with RD for two weeks. Rat estrus cycles were tested using vaginal smears. Ovarian tissues were histologically evaluated, the number of primordial, mature, and atretic follicles was calculated, and the apoptotic rate of granulosa cells. Follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2 ) levels were determined by ELISA assays. Protein levels of Forkhead Box O1 (FOXO1), KLOTHO, Bcl-2, and Bax were investigated in ovarian tissues of DOR rats. The binding between FOXO1 and KLOTHO was verified by ChIP assay. High-dose administration of RD into DOR rats improved their estrus cycles, increased ovarian index, enhanced the number of primordial and mature follicles, reduced the number of atretic follicle number, and ovarian granulosa cell apoptosis in addition to inhibiting FSH and LH levels and upregulating E2 expression. FOXO1 and KLOTHO were significantly suppressed in DOR rats. FOXO1 knockdown partially suppressed the protective effects of RD on DOR rats, and KLOTHO overexpression could restore RD-induced blockade of DOR development despite knocking down FOXO1. FOXO1 antibody enriched KLOTHO promoter, and the binding between them was reduced in DOR group compared to that in sham group. RD improved ovarian functions in DOR rats and diminished granulosa cell apoptosis via the FOXO1/KLOTHO axis.
Keyword
Figure
Reference
-
1. Zhang QL, Lei YL, Deng Y, Ma RL, Ding XS, Xue W, Sun AJ. 2022; Treatment progress in diminished ovarian reserve: Western and Chinese medicine. Chin J Integr Med. doi: 10.1007/s11655-021-3353-2. [Epub ahead of print]. DOI: 10.1007/s11655-021-3353-2. PMID: 35015221. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85122748390&origin=inward.
Article2. Park SU, Walsh L, Berkowitz KM. 2021; Mechanisms of ovarian aging. Reproduction. 162:R19–R33. DOI: 10.1530/REP-21-0022. PMID: 33999842. PMCID: PMC9354567. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85110943330&origin=inward.
Article3. Buigues A, Marchante M, Herraiz S, Pellicer A. 2020; Diminished ovarian reserve chemotherapy-induced mouse model: a tool for the preclinical assessment of new therapies for ovarian damage. Reprod Sci. 27:1609–1619. Erratum in: Reprod Sci. 2021;28:615. DOI: 10.1007/s43032-020-00191-w. PMID: 32430713. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084993228&origin=inward.
Article4. Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. 2018; Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 35:17–23. DOI: 10.1007/s10815-017-1058-4. PMID: 28971280. PMCID: PMC5758472. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030471235&origin=inward.
Article5. Murase T, Iwase A, Komatsu K, Bayasula , Nakamura T, Osuka S, Takikawa S, Goto M, Kotani T, Kikkawa F. 2018; Follicle dynamics: visualization and analysis of follicle growth and maturation using murine ovarian tissue culture. J Assist Reprod Genet. 35:339–343. DOI: 10.1007/s10815-017-1073-5. PMID: 29080194. PMCID: PMC5845041. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85032469760&origin=inward.
Article6. Fan Y, Chang Y, Wei L, Chen J, Li J, Goldsmith S, Silber S, Liang X. 2019; Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. J Assist Reprod Genet. 36:1225–1235. DOI: 10.1007/s10815-019-01446-5. PMID: 30980221. PMCID: PMC6602993. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064352322&origin=inward.
Article7. Liu W, Chen Q, Liu Z, Weng Z, Nguyen TN, Feng J, Zhou S. 2021; Zihuai recipe alleviates cyclophosphamide-induced diminished ovarian reserve via suppressing PI3K/AKT-mediated apoptosis. J Ethnopharmacol. 277:113789. DOI: 10.1016/j.jep.2021.113789. PMID: 33422655. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85106926813&origin=inward.8. Lim SW, Jin L, Luo K, Jin J, Shin YJ, Hong SY, Yang CW. 2017; Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 8:e2972. DOI: 10.1038/cddis.2017.365. PMID: 28771227. PMCID: PMC5596554. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85026836651&origin=inward.
Article9. Wu Z, He Q, Zeng B, Zhou H, Zhou S. 2020; Juvenile hormone acts through FoxO to promote Cdc2 and Orc5 transcription for polyploidy-dependent vitellogenesis. Development. 147:dev188813. DOI: 10.1242/dev.188813. PMID: 32907849. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85091585479&origin=inward.10. Herndon MK, Law NC, Donaubauer EM, Kyriss B, Hunzicker-Dunn M. 2016; Forkhead box O member FOXO1 regulates the majority of follicle-stimulating hormone responsive genes in ovarian granulosa cells. Mol Cell Endocrinol. 434:116–126. DOI: 10.1016/j.mce.2016.06.020. PMID: 27328024. PMCID: PMC4983523. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84976493060&origin=inward.
Article11. Masciangelo R, Hossay C, Chiti MC, Manavella DD, Amorim CA, Donnez J, Dolmans MM. 2020; Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J Assist Reprod Genet. 37:101–108. DOI: 10.1007/s10815-019-01628-1. PMID: 31732846. PMCID: PMC7000614. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075338703&origin=inward.
Article12. Xie T, Ye W, Liu J, Zhou L, Song Y. 2021; The emerging key role of Klotho in the hypothalamus-pituitary-ovarian axis. Reprod Sci. 28:322–331. DOI: 10.1007/s43032-020-00277-5. PMID: 32783104. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089312518&origin=inward.
Article13. Xu X, Hao Y, Zhong Q, Hang J, Zhao Y, Qiao J. 2020; Low KLOTHO level related to aging is associated with diminished ovarian reserve. Fertil Steril. 114:1250–1255. DOI: 10.1016/j.fertnstert.2020.06.035. PMID: 33153705. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85092198047&origin=inward.
Article14. Lee S, Moon JH, Song K, Taweechaipaisankul A, Jo YK, Oh HJ, Park SC, Lee BC. 2017; Establishment of transgenic porcine fibroblasts expressing a human Klotho gene and its effects on gene expression and preimplantation development of cloned embryos. DNA Cell Biol. 36:42–49. DOI: 10.1089/dna.2016.3482. PMID: 28004977. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85008689698&origin=inward.
Article15. Xing L, Fang J, Zhu B, Wang L, Chen J, Wang Y, Huang J, Wang H, Yao X. 2021; Astragaloside IV protects against podocyte apoptosis by inhibiting oxidative stress via activating PPARγ-Klotho-FoxO1 axis in diabetic nephropathy. Life Sci. 269:119068. DOI: 10.1016/j.lfs.2021.119068. PMID: 33476631. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85099664539&origin=inward.16. Yuan H, Yang M, Han X, Ni X. 2018; The therapeutic effect of the Chinese herbal medicine, Rehmanniae radix preparata, in attention deficit hyperactivity disorder via reversal of structural abnormalities in the cortex. Evid Based Complement Alternat Med. 2018:3052058. DOI: 10.1155/2018/3052058. PMID: 30405737. PMCID: PMC6204205. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85062483291&origin=inward.17. Journal of Ethnopharmacology. 2022; Retraction: Kuntai capsule attenuates premature ovarian failure through the PI3K/AKT/mTOR pathway. J Ethnopharmacol. 289:115091. Retraction of: Zhang H, Qin F, Liu A, Sun Q, Wang Q, Li Q, Lu S, Zhang D, Lu Z. J Ethnopharmacol. 2019;239:111885. DOI: 10.1016/j.jep.2022.115091. PMID: 35148932. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85124252647&origin=inward.18. Yang H, Zhai B, Wang M, Fan Y, Wang J, Cheng J, Zou J, Zhang X, Shi Y, Guo D, Tang Z. 2022; The influence of rhein on the absorption of rehmaionoside D: in vivo, in situ, in vitro, and in silico studies. J Ethnopharmacol. 282:114650. DOI: 10.1016/j.jep.2021.114650. PMID: 34536515. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85115769712&origin=inward.19. Chen L, Chen H, Lu Y, Han L, Wang S, Liu M, Li X, Zhao J, Lu C, Li S. 2020; Decoding active components in a formulation of multiple herbs for treatment of psoriasis based on three cell lines fishing and liquid chromatography-mass spectrometry analysis. J Pharm Biomed Anal. 186:113331. DOI: 10.1016/j.jpba.2020.113331. PMID: 32380350. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084092130&origin=inward.
Article20. Zhou J, Pan XY, Lin J, Zhou Q, Lan LK, Zhu J, Duan R, Wang L, Sun Y, Wang L. 2022; Effects of Bushen Yiqi Huoxue Decoction in treatment of patients with diminished ovarian reserve: a randomized controlled trial. Chin J Integr Med. 28:195–201. DOI: 10.1007/s11655-020-3484-x. PMID: 33423188. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85099307929&origin=inward.
Article21. Seo HW, Cheon SM, Lee MH, Kim HJ, Jeon H, Cha DS. 2015; Catalpol modulates lifespan via DAF-16/FOXO and SKN-1/Nrf2 activation in Caenorhabditis elegans. Evid Based Complement Alternat Med. 2015:524878. DOI: 10.1155/2015/524878. PMID: 25821490. PMCID: PMC4363898. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84925310745&origin=inward.22. Jiang M, Wang W, Zhang J, Wang C, Bi Y, Li P, Yang S, Li J, Xu YT, Wang T. 2020; Protective effects and possible mechanisms of actions of Bushen Cuyun Recipe on diminished ovarian reserve induced by cyclophosphamide in rats. Front Pharmacol. 11:546. DOI: 10.3389/fphar.2020.00546. PMID: 32477106. PMCID: PMC7237638. PMID: 9aed7ae01de042b28555b813bf739929. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085371353&origin=inward.
Article23. Li X, Hu S, Zhu Q, Yao G, Yao J, Li J, Wang Y, Ding Y, Qi J, Xu R, Zhao H, Zhu Z, Du Y, Sun K, Sun Y. 2021; Addressing the role of 11β-hydroxysteroid dehydrogenase type 1 in the development of polycystic ovary syndrome and the putative therapeutic effects of its selective inhibition in a preclinical model. Metabolism. 119:154749. DOI: 10.1016/j.metabol.2021.154749. PMID: 33722534. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85103082312&origin=inward.
Article24. Sun XF, Li YP, Pan B, Wang YF, Li J, Shen W. 2018; Molecular regulation of miR-378 on the development of mouse follicle and the maturation of oocyte in vivo. Cell Cycle. 17:2230–2242. DOI: 10.1080/15384101.2018.1520557. PMID: 30244637. PMCID: PMC6226232. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053657978&origin=inward.
Article25. Jaillard S, eenivasan R Sr, Beaumont M, Robevska G, Dubourg C, Knarston IM, Akloul L, van den Bergen J, Odent S, Croft B, Jouve G, Grover SR, Duros S, Pimentel C, Belaud-Rotureau MA, Ayers KL, Ravel C, Tucker EJ, Sinclair AH. 2020; Analysis of NR5A1 in 142 patients with premature ovarian insufficiency, diminished ovarian reserve, or unexplained infertility. Maturitas. 131:78–86. DOI: 10.1016/j.maturitas.2019.10.011. PMID: 31787151. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075133320&origin=inward.
Article26. Zhang C, Xu X. 2016; Advancement in the treatment of diminished ovarian reserve by traditional Chinese and Western medicine. Exp Ther Med. 11:1173–1176. DOI: 10.3892/etm.2016.3025. PMID: 27073418. PMCID: PMC4812125. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84958755721&origin=inward.
Article27. Cagnacci A, Venier M. 2019; The controversial history of hormone replacement therapy. Medicina (Kaunas). 55:602. DOI: 10.3390/medicina55090602. PMID: 31540401. PMCID: PMC6780820. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072516149&origin=inward.
Article28. Christodoulaki A, Boel A, Tang M, De Roo C, Stoop D, Heindryckx B. 2021; Prospects of germline nuclear transfer in women with diminished ovarian reserve. Front Endocrinol (Lausanne). 12:635370. DOI: 10.3389/fendo.2021.635370. PMID: 33692760. PMCID: PMC7937897. PMID: 423211a96efe4826b5e0e36efcca6f85. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85102366231&origin=inward.
Article29. Peng H, Zeng L, Zhu L, Luo S, Xu L, Zeng L, Li J, Liang Q, Geng H. 2019; Zuogui Pills inhibit mitochondria-dependent apoptosis of follicles in a rat model of premature ovarian failure. J Ethnopharmacol. 238:111855. DOI: 10.1016/j.jep.2019.111855. PMID: 30953821. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064530336&origin=inward.
Article30. Yang H, Fan Y, Cheng J, Zou J, Zhang X, Shi Y, Guo D. 2020; Network pharmacology-based prediction of active ingredients and potential targets of ShengDiHuang Decoction for treatment of dysfunctional uterine bleeding. Evid Based Complement Alternat Med. 2020:7370304. DOI: 10.1155/2020/7370304. PMID: 32454870. PMCID: PMC7240676. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085903084&origin=inward.
Article31. Xu Z, Dai XX, Zhang QY, Su SL, Yan H, Zhu Y, Shang EX, Qian DW, Duan JA. 2020; Protective effects and mechanisms of Rehmannia glutinosa leaves total glycoside on early kidney injury in db/db mice. Biomed Pharmacother. 125:109926. DOI: 10.1016/j.biopha.2020.109926. PMID: 32028239. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078757862&origin=inward.
Article32. Fu C, Wu Y, Liu S, Luo C, Lu Y, Liu M, Wang L, Zhang Y, Liu X. 2022; Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J Ethnopharmacol. 289:115021. DOI: 10.1016/j.jep.2022.115021. PMID: 35091012. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85123796044&origin=inward.33. Wei C, Xiang S, Yu Y, Song J, Zheng M, Lian F. 2021; miR-221-3p regulates apoptosis of ovarian granulosa cells via targeting FOXO1 in older women with diminished ovarian reserve (DOR). Mol Reprod Dev. 88:251–260. DOI: 10.1002/mrd.23457. PMID: 33694202. PMCID: PMC8251591. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85102460770&origin=inward.
Article34. Rehnitz J, Capp E, Messmer B, Nguyen XP, Germeyer A, Freis A, Dietrich JE, Hinderhofer K, Strowitzki T, Vogt PH. 2021; FMR1 and AKT/mTOR signaling in human granulosa cells: functional interaction and impact on ovarian response. J Clin Med. 10:3892. DOI: 10.3390/jcm10173892. PMID: 34501340. PMCID: PMC8432207. PMID: 1eddecfc559842628b4ebe4b375ffc0e. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85113900484&origin=inward.
Article35. Liu T, Zhao H, Wang J, Shu X, Gao Y, Mu X, Gao F, Liu H. 2017; The role of fructose-1,6-bisphosphatase 1 in abnormal development of ovarian follicles caused by high testosterone concentration. Mol Med Rep. 16:6489–6498. DOI: 10.3892/mmr.2017.7463. PMID: 28901488. PMCID: PMC5865816. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030121314&origin=inward.
Article36. Kuro-O M. 2019; The Klotho proteins in health and disease. Nat Rev Nephrol. 15:27–44. DOI: 10.1038/s41581-018-0078-3. PMID: 30455427. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056791142&origin=inward.
Article37. Liu T, Liu Y, Huang Y, Chen J, Yu Z, Chen C, Lai L. 2019; miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic Biol Med. 141:383–392. DOI: 10.1016/j.freeradbiomed.2019.07.010. PMID: 31310795. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85068932302&origin=inward.
Article38. Xu L, Idrees M, Joo MD, Sidrat T, Wei Y, Song SH, Lee KL, Kong IK. 2021; Constitutive expression of TERT enhances β-Klotho expression and improves age-related deterioration in early bovine embryos. Int J Mol Sci. 22:5327. DOI: 10.3390/ijms22105327. PMID: 34070219. PMCID: PMC8158768. PMID: 26d10fb610cd491e89911976bb17aae4. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85105880535&origin=inward.
Article39. Jin J, Jin L, Lim SW, Yang CW. 2016; Klotho deficiency aggravates tacrolimus-induced renal injury via the phosphatidylinositol 3-kinase-Akt-Forkhead box protein O pathway. Am J Nephrol. 43:357–365. DOI: 10.1159/000446447. PMID: 27174564. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84967104385&origin=inward.
Article40. Gong Y, Luo S, Fan P, Zhu H, Li Y, Huang W. 2020; Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod Biol Endocrinol. 18:121. DOI: 10.1186/s12958-020-00677-x. PMID: 33287836. PMCID: PMC7720521. PMID: 68321e5ae7da402a8d0ad9c414601cba. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85097218885&origin=inward.
Article41. Ma K, Chen Y, Fan X, Yuan Y, Wang K, Tian C, Li M. 2020; Dingkun Pill replenishes diminished ovarian reserve through the PI3K/AKT/mTOR signaling pathway in TWP-induced mice. J Ethnopharmacol. 262:112993. DOI: 10.1016/j.jep.2020.112993. PMID: 32473368. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089796889&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Homeostatic Changes in Salivary Gland Acinar Cells in Primary Sjöögren's Syndrome: A Review
- Role of Klotho and N-acetylcysteine in Oxidative Stress Associated with the Vitrification of Ovarian Tissue Cytoprotective Function of Klotho in Cryopreservation
- In vitro fertilization outcomes in women with surgery induced diminished ovarian reserve after endometrioma operation: Comparison with diminished ovarian reserve without ovarian surgery
- MiR-542-5p Inhibits Hyperglycemia and Hyperlipoidemia by Targeting FOXO1 in the Liver
- Prevalence of Foxp3 Positive T Regulatory Cells is Increased during Progression of Cutaneous Squamous Tumors